2,5-二甲氧基苯乙腈 | 18086-24-3
中文名称
2,5-二甲氧基苯乙腈
中文别名
2,4-二甲氧基苯酚
英文名称
2-(2,5-dimethoxyphenyl)acetonitrile
英文别名
2,5-dimethoxyphenylacetonitrile
CAS
18086-24-3
化学式
C10H11NO2
mdl
MFCD00016388
分子量
177.203
InChiKey
DBKDGRJAFWDOOJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:51-53°C
-
沸点:167 °C(Press: 10 Torr)
-
密度:1.082±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:42.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:TOXIC
-
危险品标志:T
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2926909090
-
危险品运输编号:3276
-
储存条件:| 室温 |
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,5-二甲氧基苯基乙酸 (2,5-dimethoxyphenyl)acetic acid 1758-25-4 C10H12O4 196.203 2,5-二甲氧基甲苯 2,5-dimethoxy toluene 24599-58-4 C9H12O2 152.193 2,5-二甲氧基苄溴 2,5-dimethoxybenzyl bromide 60732-17-4 C9H11BrO2 231.089 2,5-二甲氧基苄氯 2,5-dimethoxybenzyl chloride 3840-27-5 C9H11ClO2 186.638 2,5-二甲氧基苯甲醛 2,5-dimethoxybenzaldehyde 93-02-7 C9H10O3 166.177 对二甲氧基苯甲醇 2,5-dimethoxybenzyl alcohol 33524-31-1 C9H12O3 168.192 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(2,5-dimethoxyphenyl)-2-methylpropanenitrile 23023-26-9 C12H15NO2 205.257 —— 2-(2,5-dimethoxyphenyl)ethylbromide 99187-42-5 C10H13BrO2 245.116 2,5-二甲氧基苯乙胺 2,5-dimethoxyphenethylamine 3600-86-0 C10H15NO2 181.235 2,5-二甲氧基苯乙醇 2-(2,5-dimethoxyphenyl)ethanol 7417-19-8 C10H14O3 182.219 2,5-二甲氧基苯基乙酸 (2,5-dimethoxyphenyl)acetic acid 1758-25-4 C10H12O4 196.203 —— 2-(2,5-dimethoxy-phenyl)-4-dimethylamino-butyronitrile 231289-63-7 C14H20N2O2 248.325 2,5-二甲氧苯基乙酸乙酯 ethyl (2,5-dimethoxyphenyl)acetate 66469-86-1 C12H16O4 224.257
反应信息
-
作为反应物:参考文献:名称:羟色胺。第一部分。丁苯丁宁,6-羟基丁苯丁宁和5-羟色胺摘要:DOI:10.1039/jr9540001165
-
作为产物:描述:参考文献:名称:Approaches to anthracyclines. 1. Conjugate aroylation of .alpha.,.beta.-unsaturated esters摘要:DOI:10.1021/jo01301a015
文献信息
-
Fluorinated biphenyls from aromatic arylations with pentafluorobenzenediazonium and related cations. Competition between arylation and azo coupling作者:Dmitry Kosynkin、T. Michael Bockman、Jay K. KochiDOI:10.1039/a701745f日期:——High yields of the mixed perfluorinated biaryls (C6F5–Ar) are obtained by the catalytic dediazoniation of the pentafluorobenzenediazonium salt (C6F5N2+BF4–) in acetonitrile solutions containing various aromatic substrates (ArH) together with small amounts of iodide salts. Activated (electron-rich) as well as deactivated (electron-poor) arenes are successfully pentafluorophenylated by this method. The arylation is distinct from the azo coupling of the same substrates, which takes place in the absence of the iodide catalyst and yields the corresponding diazene (C6F5NN–Ar) as product. The catalytic role of iodide, and the isomeric product distributions obtained with this procedure indicate that the arylation proceeds via the pentafluorophenyl radical in a efficient homolytic chain process. Since azo coupling involves electrophilic aromatic substitution of electron-rich ArH by C6F5N2+, the two competing pathways are distinct and do not have reactive intermediates in common.通过催化脱重氮化反应,在含有各种芳香族底物(ArH)及少量碘化盐的乙腈溶液中,五氟苯重氮盐( N2+BF4–)能够高效生成混合全氟联苯(C6F5–Ar),产率较高。无论是活化的(电子富集的)还是去活化的(电子贫乏的)芳香族化合物,都能通过此方法成功实现五氟苯基化。这种芳基化反应与同种底物的偶氮耦合反应截然不同,后者在无碘催化剂存在下进行,产物为相应重氮烯( NN–Ar)。碘化物在催化中的作用以及由此过程获得的不同异构产物分布表明,芳基化反应是通过高效的均裂链式过程,经由五氟苯基自由基进行的。由于偶氮耦合涉及电子富集的ArH通过亲电芳香取代反应与 N2+的结合,这两种竞争路径是不同的,且没有共同的反应中间体。
-
Preparation of Indolenines via Nucleophilic Aromatic Substitution作者:Florian Huber、Joel Roesslein、Karl GademannDOI:10.1021/acs.orglett.9b00489日期:2019.4.19An unusual aromatic substitution to access indolenines is described. 2-(2-Methoxyphenyl)acetonitrile derivatives are reacted with various alkyl and aryl Li reagents to furnish the corresponding indolenine products, constituents of natural products, and cyanine dyes such as indocyanine green. This new method was used to synthesize 41 indolenines with large functional group tolerance, and selected examples
-
Domino Carbocationic Cyclization of Functionalized Cyclopropyl Ketones: Facile One-Pot Access to Peri- and Angularly Fused Polycyclic Aromatic and Heteroaromatic Frameworks作者:Sukumar Nandi、U. K. Syam Kumar、Hiriyakkanavar Ila、Hiriyakkanavar JunjappaDOI:10.1021/jo020230r日期:2002.7.1yields in a one-pot operation. The methodology provides efficient, high-yield routes for synthesis of novel substituted dihydrophenalenes, dihydrobenzo[d,e]anthracene, cyclopenta[a]naphthalene, and fused heteroaromatics such as substituted 4,5-dihydrobenzo[c,d]indole, dihydronaphtho[1,8-b,c]thiophene, dihydroindeno[5,4-b]- and -[4,5-b]-thiophenes, cyclopenta[a]carbazole, and dihydrocyclopenta[e]indazol-3-one已显示通过碱诱导的各种芳基/杂芳基乙腈与1-(2-芳基环丙基)-3,3-(双甲硫基)-2-丙烯-1-酮的1,4-加成消除反应得到的共轭加合物很容易合成一锅操作中,酸诱导的多米诺碳正离子重排可高产率产生各种取代的三环芳族和杂芳族骨架。该方法为合成新型取代的二氢菲咯啉,二氢苯并[d,e]蒽,环戊[a]萘和稠合的杂芳族化合物(例如取代的4,5-二氢苯并[c,d]吲哚,二氢萘[])提供了有效的高产率途径。 1,8-b,c]噻吩,二氢茚并[5,4-b]-和-[4,5-b]噻吩,环戊[a]咔唑和二氢环戊[e]吲唑-3-one衍生物。这种有趣的多米诺骨牌过程的可能机理似乎涉及逐步或伴随的酸诱导的开环和环丙基酮的分子内环缩合反应,从而生成带有反应性苄基碳正离子的苯并稠合的芳烃(或杂芳烃)中间体,该中间体被预先存在的芳香族分子内捕获(或杂芳族)环或新形成的苯环,分别得到周边稠合或成角度稠合的产物。因此,整个
-
Degradation of 3-Aryl-2-hydroxyiminopropionic Acids into Arylacetonitriles Using 1,1'-Carbonyldiimidazole or 2,2'-Oxalyldi(o-sulfobenzimide).作者:Tokujiro KITAGAWA、Megumi KAWAGUCHI、Sachie INOUE、Shinji KATAYAMADOI:10.1248/cpb.39.3030日期:——1, 1'-Carbonyldiimidazole (1) is a useful reagent for the preparation of arylacetonitriles (9) from 3-aryl-2-hydroxyiminopropionic acids (8), and 2, 2'-oxalyldi (o-sulfobenzimide) (2) can also be used for this purpose under essentially neutral conditions.
-
α-Oxoketene dithioacetal mediated aromatic annulation: highly efficient and concise synthetic routes to potentially carcinogenic polycyclic aromatic hydrocarbons作者:Sukumar Nandi、Kausik Panda、J.R Suresh、Hiriyakkanavar Ila、Hiriyakkanavar JunjappaDOI:10.1016/j.tet.2004.02.053日期:2004.4Highly efficient regiospecific routes to potentially carcinogenic polycyclic aromatic hydrocarbons such as substituted benzo[c]phenanthrenes, benzo[c]fluorenes, 16,17-dihydro-11-methyl-15[H]cyclopenta[a]phenanthrene, 5-methyl-7,8,9,10-tetrahydrochrysene and 1,4-dimethylphenanthrene have been developed. The overall strategy involves our aromatic annulation protocol through base induced conjugate addition–elimination
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
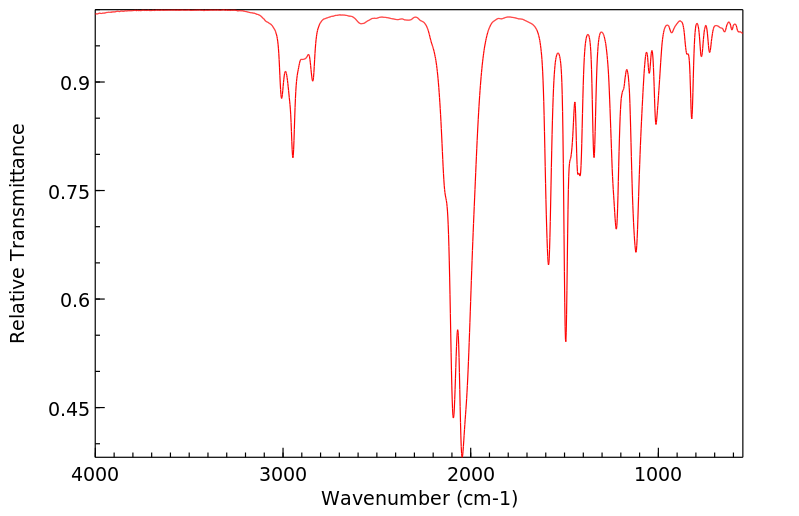
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫