3-butyl-1H-benzo[4,5]imidazo[1,2-a][1,3,5]triazine-2,4-dione | 41136-38-3
中文名称
——
中文别名
——
英文名称
3-butyl-1H-benzo[4,5]imidazo[1,2-a][1,3,5]triazine-2,4-dione
英文别名
3-Butyl-2,4-dioxo-1,2,3,4-tetrahydro-s-triazino-[1,2-a]-benzimidazole;3-Butyl-2,4-dioxo-1,2,3,4-tetrahydro-s-triazino<1,2-a>benzimidazol;3-Butyl2,4-dioxo-<1,2-a>-s-triazinobenzimidazol;Butyl-3-dioxo-2,4-s-triazino<1,2-a>benzimidazol;3-Butyl-2,4-dioxo(1,2-a)-s-triazinobenzimidazole;3-butyl-1H-[1,3,5]triazino[1,2-a]benzimidazole-2,4-dione
CAS
41136-38-3
化学式
C13H14N4O2
mdl
——
分子量
258.28
InChiKey
BMENGGSVYVKZLZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:285 °C
-
沸点:387.7±25.0 °C(Predicted)
-
密度:1.42±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:19
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.31
-
拓扑面积:67.2
-
氢给体数:1
-
氢受体数:3
SDS
反应信息
-
作为产物:参考文献:名称:Preparation of摘要:公式为 ##STR1## 的2,4-二氧代-1,2,3,4-四氢-s-三氮唑-[1,2-a]-苯并咪唑可通过将公式为 ##STR2## 的苯并咪唑化合物与公式为 ##STR3## 的二苯基碳酸酯反应制得,其中R.sup.1是可选的取代基的烷基或芳基,烯基或二烷基氨基,R.sup.2是氢,烷基或三氟甲基,R.sup.3是任何R.sup.1基团或羟基羰基烷基,R.sup.4是氢,烷基或卤素。该反应可以在容器中使用未纯化的苯并咪唑进行,无需分离。其中R.sup.1为各种ω-取代烷基基团,R.sup.2为氢或甲基的化合物是新的,所有化合物均表现出杀真菌活性。公开号:US04053598A1
-
作为试剂:描述:2-氨基苯并咪唑 、 碳酸二苯酯 、 苯酚 、 二正丁胺 、 1-(2-Benzimidazolyl)-3-n-butyl-harnstoff 、 potassium carbonate 在 二丁醚 、 3-butyl-1H-benzo[4,5]imidazo[1,2-a][1,3,5]triazine-2,4-dione 、 crude product 、 氢氧化钾 、 水 作用下, 以 苯甲腈 、 吡啶 为溶剂, 反应 37.5h, 以3-Butyl-2,4-dioxo-1,2,3,4-tetrahydro-s-triazino-[1,2-a]-benzimidazole was isolated from the filtrate by acidification to a pH value of about 5的产率得到3-butyl-1H-benzo[4,5]imidazo[1,2-a][1,3,5]triazine-2,4-dione参考文献:名称:Preparation of摘要:化学式为##STR1##的2,4-Dioxo-1,2,3,4-tetrahydro-s-triazino-[1,2-a]-benzimidazoles是通过将化学式为##STR2##的苯并咪唑化合物与化学式为##STR3##的二苯基碳酸酯反应而制得的。其中,R.sup.1是可选的取代烷基或芳基、烯基或二烷基氨基,R.sup.2是氢、烷基或三氟甲基,R.sup.3是任何一个R.sup.1基团或羟基羧酸烷基,R.sup.4是氢、烷基或卤素。该反应可以在含有未纯化苯并咪唑的容器中进行,无需分离。其中,R.sup.1为各种ω-取代烷基基团,R.sup.2为氢或甲基的化合物是新的,所有化合物均具有杀菌活性。公开号:US04053598A1
文献信息
-
MAYER K. H.; LAUERER D.; HEITZER H., SYNTHESIS <SYNT-BF>, 1977, NO 11, 804-806作者:MAYER K. H.、 LAUERER D.、 HEITZER H.DOI:——日期:——
-
US4053598A申请人:——公开号:US4053598A公开(公告)日:1977-10-11
表征谱图
-
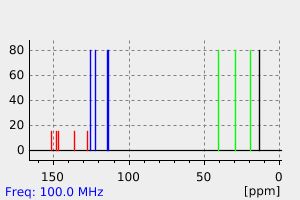
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
麦穗宁
马哌斯汀
颜料橙62
顺式-5,6-二氢-4,5-二甲基-4H-咪唑并[1,5,4-De]喹喔啉
韦罗肟
青菌灵
雷贝拉唑钠
雷贝拉唑硫醚N-氧化物
雷贝拉唑砜 N-氧化物
雷贝拉唑砜
雷贝拉唑杂质2
雷贝拉唑 N-氧化物
雷贝拉唑
阿苯达唑砜
阿苯达唑杂质L
阿苯达唑杂质J(EP)
阿苯达唑杂质J
阿苯达唑杂质F
阿苯达唑杂质14
阿苯达唑杂质13
阿苯达唑亚砜
阿苯达唑
阿苯哒唑砜-D3
阿苯哒唑-D3
阿地本旦
阿司咪唑-d3
阿司咪唑
钠4-[5-氯-2-[(E,3E)-3-[6-氯-1-乙基-3-(4-磺酸丁基)-5-(三氟甲基)苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-6-(三氟甲基)苯并咪唑-1-鎓-1-基]丁烷-1-磺酸盐
邻甲磺酰胺基苯乙酸
那地特罗
达比加群酯杂质M
达比加群酯杂质4
达比加群酯杂质1
达比加群酯杂质
达比加群酯N-氧化物
达比加群酯
达比加群脂杂质10
达比加群甲酯杂质
达比加群杂质J
达比加群杂质J
达比加群杂质F
达比加群杂质E
达比加群杂质D
达比加群杂质C5
达比加群杂质38
达比加群杂质13
达比加群杂质10(DABRC-10)
达比加群杂质10