烯丙基丙基硫醚 | 27817-67-0
中文名称
烯丙基丙基硫醚
中文别名
烯丙基-N-丙基硫醚;烯丙基二硫化物;丙基烯丙基硫醚;3-(丙基硫代)-1-丙烯;烯丙基-n-丙基硫醚
英文名称
allyl propyl sulfide
英文别名
allyl-n-propyl suldide;1-prop-2-enylsulfanylpropane
CAS
27817-67-0
化学式
C6H12S
mdl
MFCD00015220
分子量
116.227
InChiKey
JMLIYQJQKZYHDQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:140°C
-
密度:0,87 g/cm3
-
LogP:2.549 (est)
-
保留指数:825;858;899
-
稳定性/保质期:
存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:7
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:3
-
危险类别码:R36/37/38
-
安全说明:S26,S36/37/39
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : ALLYL PROPYL SULFIDE
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 27817-67-0
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Formula : C6H12S
Molecular Weight : 116,23 g/mol
CAS-No. : 27817-67-0
No components need to be disclosed according to the applicable regulations.
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, Sulphur oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Avoid breathing vapours, mist or gas. Ensure adequate ventilation.
For personal protection see section 8.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the
environment must be avoided.
Methods and materials for containment and cleaning up
Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Normal measures for preventive fire protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection not required. For nuisance exposures use type OV/AG (US) or type ABEK
(EU EN 14387) respirator cartridges. Use respirators and components tested and approved under
appropriate government standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into
the environment must be avoided.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 2,837
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
Toxic to aquatic life.
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Further information
Copyright 2013 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
反应信息
-
作为反应物:描述:参考文献:名称:含硫氨基酸的光化学和辐射化学。1-丙烯基噻吩基的新反应。摘要:DOI:10.1021/jo00899a011
-
作为产物:描述:Dithiocarbonic acid S-allyl ester S-propyl ester 在 四(三苯基膦)钯 1,2-双(二苯基膦)乙烷 作用下, 以 乙醚 为溶剂, 反应 8.0h, 以54%的产率得到烯丙基丙基硫醚参考文献:名称:Palladium-Catalyzed Synthesis of Allylic and Benzylic Sulfides from the Corresponding Dithiocarbonates摘要:烯丙基和苄基硫化物是在存在钯(0)-膦配合物催化剂的条件下,由O-(2-烯烃基)或S-(2-烯烃基) S-烷基和O-苄基 S-烷基二硫氨基碳酸酯制备的。DOI:10.1055/s-1987-27849
-
作为试剂:描述:溶剂黄146 在 1-vinyl-3-butylimidazolium chloride 、 methylvinylcyclosiloxane 、 烯丙基丙基硫醚 、 magnesium diacrylate 、 sodium hydroxide 作用下, 反应 2.58h, 以98.9%的产率得到sodium acetate acetic acid参考文献:名称:一种饲料添加剂双乙酸钠的制备方法摘要:本发明涉及农业助剂制备领域,具体关于一种饲料添加剂双乙酸钠的制备方法;本发明采用醋酸和烧碱为原料的无溶剂一步法合成双乙酸钠,相对其他方法,具有工艺操作简单,无溶剂回收流程,无三废排放的优点。公开号:CN113045402A
文献信息
-
Stereoselective synthesis of 1,4-disubstituted 1,3-diene from aldehyde using organotitanium reagent作者:Yoshihiko Ikeda、Junzo Ukai、Nobuo Ikeda、Hisashi YamamotoDOI:10.1016/s0040-4020(01)90007-9日期:——Organotitanium reagent generated from 1-t-butylthio-3-trimethylsilyl-1-propene condenses with aldehydes to give 1 -t-butylthio-(E,Z)-1,3-alkadienes in a single step via (E)-erythro-β-hydroxysilane in a highly regio- and stereoselective manner. 1,4-Dialkyl-1,3-diene is obtaind from the diene sulfide by cross coupling reaction with Grignard reagent in the presence of nickel catalyst. The utility of the
-
Binuclear manganese complexes as catalysts in the selective and efficient oxidation of sulfides to sulfones作者:Derek H.R. Barton、Wenge Li、Jason A. SmithDOI:10.1016/s0040-4039(98)01518-4日期:1998.9The binuclear MnIVMnIV manganese complex 1 catalyzes the periodic acid oxidation of sulfides to sulfones under mild conditions. The reaction was found to be highly selective giving almost quantitative yields of the sulfones even in the presence other easily oxidized groups. Only amines were found to hinder the reaction.
-
Highly Efficient and Stereoselective Thioallylation of Alkynes: Possible Gold Redox Catalysis with No Need for a Strong Oxidant作者:Jin Wang、Shuyao Zhang、Chang Xu、Lukasz Wojtas、Novruz G. Akhmedov、Hao Chen、Xiaodong ShiDOI:10.1002/anie.201802540日期:2018.6.4Stereoselective thioallylation of alkynes under possible gold redox catalysis was accomplished with high efficiency (as low as 0.1 % catalyst loading, up to 99 % yield) and broad substrate scope (various alkynes, inter‐ and intramolecular fashion). The gold(I) catalyst acts as both a π‐acid for alkyne activation and a redox catalyst for AuI/III coupling, whereas the sulfonium cation generated in situ
-
The catalytic reactions of triethyl- and triethoxy-silane with unsaturated sulphides作者:M.G. Voronkov、N.N. Vlasova、S.A. Bolshakova、S.V. KirpichenkoDOI:10.1016/s0022-328x(00)90626-0日期:1980.5di-alkenyl sulphides of the type RS(CH2)nCHCH2 (R = C2H5, CH2CH, CH2CHCH2, C3H7, n = 0, 1 and 4) by triethyl- and triethoxy-silane, catalyzed by H2PtCl6·6 H2O, (Ph3P)3RhCl and (PhCN)2PdCl2·Ph3P, has been studied. The addition of hydrosilane to the double bond of alkenyl sulphide leads to a mixture of two isomeric monoadducts. The hydrosilane can cleave the CS bond of the initial sulphides giving the该类型的RS的单-和二-烯基硫化物的氢化硅烷化(CH 2)Ñ CHCH 2(R = C 2 H ^ 5,CH 2 CH,CH 2 CHCH 2,C 3 ħ 7,Ñ = 0 H 1 PtCl 6 ·6 H 2 O,(Ph 3 P)3 RhCl和(PhCN)2 PdCl 2 ·Ph 3催化三乙基-和三乙氧基-硅烷(1和4)P,已被研究。在链烯基硫的双键上加氢硅烷会导致两种异构单加合物的混合物。氢硅烷可以切割初始硫化物给予thiosilanes的相应衍生物的CS键,X 3 SIS(CH 2)Ñ CHCH 2(X = C 2 H ^ 5,C 2 H ^ 5 O)。烯基硫的氢化硅烷化伴随着一些副反应,例如脱氢缩合,还原和聚合。研究了催化剂性质,氢化硅烷和链烯基硫化物的结构对反应路线的影响。
-
Imino‐λ <sup>3</sup> ‐iodane and Catalytic Amount of I <sub>2</sub> ‐Mediated Synthesis of <i>N</i> ‐Allylsulfenamides via [2,3]‐Sigmatropic Rearrangement作者:Cody L. Makitalo、Akira Yoshimura、Gregory T. Rohde、Irina A. Mironova、Rosa Y. Yusubova、Mekhman S. Yusubov、Viktor V. Zhdankin、Akio SaitoDOI:10.1002/ejoc.202000961日期:2020.11.8Imino‐λ3‐iodane and catalytic I2‐mediated facile metal‐free [2,3]‐sigmatropic rearrangement reaction of allyl sulfides producing N‐allysulfenamiides has been developed
表征谱图
-
氢谱1HNMR
-
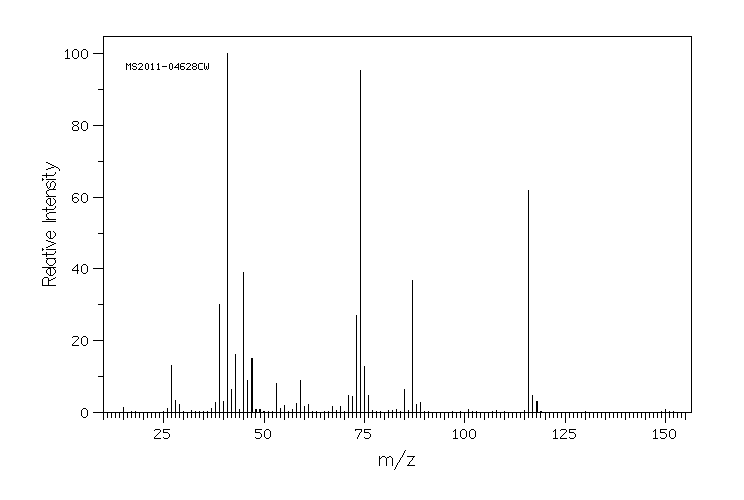
质谱MS
-
碳谱13CNMR
-
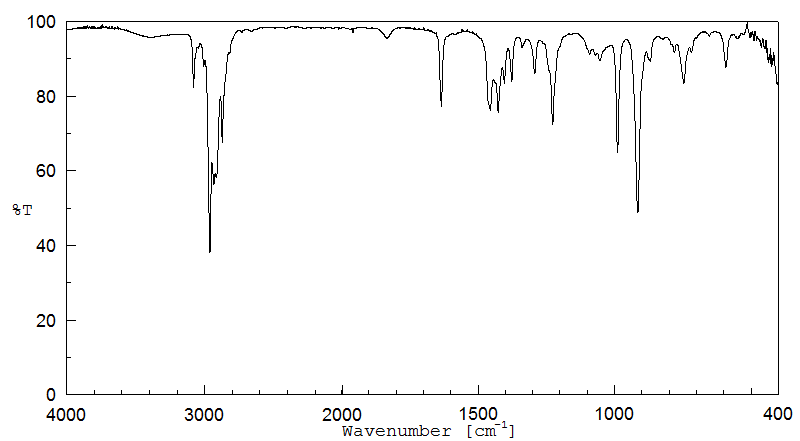
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
烯丙硫醇
烯丙基硫氰酸酯
烯丙基甲基硫醚
烯丙基甲基二硫醚
烯丙基异丙基硫醚
烯丙基仲丁基硫醚
烯丙基仲丁基硫醚
烯丙基乙基巯醚
烯丙基丙基硫醚
烯丙基丙基二硫醚
烯丙基丁基碳o三硫代酸酯
烯丙基2-氯乙基硫醚
烯丙基(环己-3-烯-1-基)硫烷
烯丙基(2,2-二乙氧基乙基)硫烷
二烯丙基硫醚
二烯丙基四硫醚
二烯丙基二硫
乙基烯丙基二硫醚
三硫代碳酸二-2-丙烯基酯
三甲基甲硅烷基3-[(三甲基甲硅烷基)氧代]癸酸酯
[二(丙-2-烯基硫基)亚甲基氨基]氢氰酸
S-烯丙基硫代氯甲酸酯
S-烯丙基-O,O-二乙基硫代磷酸酯
3-[(2,2-二甲氧基乙基)硫基]-1-丙烯
3-(丙-2-烯基五硫烷基)丙-1-烯
2-乙基-2-烯丙基巯基丁酰氯
2-(烯丙基硫代)丙酸
2-(全氟辛基)乙基丙烯基硫化物
1-乙烯基硫代-3-烯丙基硫代-1-丙烯
1-(烯丙基硫基)己烷
(E)-烯丙基1-丙烯基硫化物
2-methylsulfanyl-1-methylsulfanylmethyl-ethyl
3-(1,2,2-Trichloro-propylsulfanyl)-propene
(3-Allylsulfanyl-propyl)-dimethyl-silane
diallyl dithioether
Allyl-cyclopropylmethyl-sulfid
4-Allylsulfanyl-non-1-en-8-yn-5-one
(2-Allylsulfanyl-1-methyl-ethyl)-diethyl-silane
Dithiophosphoric acid S,S'-diallyl ester O-ethyl ester
4-cyclohexylthio-1,2-butadiene
(4S)-4-[(1S)-1-azido-2,2-difluorobut-3-enyl]-2,2-dimethyl-1,3-dioxolane
Dimethyl<(methylthio)methylen>ammonium-triiodomercurat
1,1-dibromo-3-hexylsulfanyl-pent-1-ene
2-adamantyl allyl sulfide
(S)-1,1-dibromo-3-hexylsulfanyl-pent-1-ene
μ-disulfido-1,2-dithio-dicarbonic acid bis-allylamide
thiocyanato-acetaldehyde
cis-1,3-bis(allylthio)cyclobutane