2-乙基-1-戊醇 | 27522-11-8
中文名称
2-乙基-1-戊醇
中文别名
2-乙基戊醇
英文名称
2-ethyl-1-pentanol
英文别名
2-ethylpentan-1-ol
CAS
27522-11-8
化学式
C7H16O
mdl
——
分子量
116.203
InChiKey
UKFQWAVMIMCNEH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-30.45°C (estimate)
-
沸点:166°C
-
密度:0.8290
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905199090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 Alpha-乙基戊酸 2-ethylvaleric acid 20225-24-5 C7H14O2 130.187 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 Alpha-乙基戊酸 2-ethylvaleric acid 20225-24-5 C7H14O2 130.187
反应信息
-
作为反应物:描述:2-乙基-1-戊醇 在 E. coli perisplasmic aldehyde oxidase 、 F. graminearum galactose oxidase 、 catalase 作用下, 以 aq. phosphate buffer 、 乙腈 为溶剂, 反应 10.0h, 生成 Alpha-乙基戊酸参考文献:名称:Enzyme cascade reactions: synthesis of furandicarboxylic acid (FDCA) and carboxylic acids using oxidases in tandem摘要:三种酶在温和条件下结合,用于制备规模氧化HMF至FDCA和10种醇类化合物。DOI:10.1039/c5gc00707k
-
作为产物:描述:参考文献:名称:Meakin et al., Journal of Pharmacy and Pharmacology, 1959, vol. 11, p. 540,545摘要:DOI:
文献信息
-
[EN] BICYCLIC INHIBITORS OF PAD4<br/>[FR] INHIBITEURS BICYCLIQUES DE PAD4
-
Copper-Catalyzed Cross-Coupling Reaction of Organoboron Compounds with Primary Alkyl Halides and Pseudohalides作者:Chu-Ting Yang、Zhen-Qi Zhang、Yu-Chen Liu、Lei LiuDOI:10.1002/anie.201008007日期:2011.4.18Non‐activated alkyl electrophiles, including alkyl iodides, bromides, tosylates, mesylates, and even chlorides, underwent copper‐catalyzed cross‐coupling with aryl boron compounds and alkyl 9‐BBN reagents (see scheme; 9‐BBN=9‐borabicyclo[3.3.1]nonane). The reactions proceed with practically useful reactivities and thus complement palladium‐ and nickel‐catalyzed Suzuki–Miyaura coupling reactions of alkyl halides
-
[EN] PROCESSES FOR THE SYNTHESIS OF CHIRAL 1-ALKANOLS<br/>[FR] PROCÉDÉS DE SYNTHÈSE DE 1-ALCANOLS CHIRAUX申请人:PURDUE RESEARCH FOUNDATION公开号:WO2015106045A1公开(公告)日:2015-07-16The invention relates to highly enantioselective processes for the synthesis of chiral 1-alkanols via Zr-catalyzed asymmetric carboalumination of alkenes.这项发明涉及通过锆催化的不对称烯烃碳铝化反应合成手性1-烷醇的高对映选择性过程。
-
PROCESSES FOR THE SYNTHESIS OF CHIRAL 1-ALKANOLS申请人:PURDUE RESEARCH FOUNDATION公开号:US20160332940A1公开(公告)日:2016-11-17The invention relates to highly enantioselective processes for the synthesis of chiral 1-alkanols via Zr-catalyzed asymmetric carboalumination of alkenes.这项发明涉及通过Zr催化的不对称烯烃碳硼烷化反应合成手性1-烷醇的高对映选择性过程。
-
PROCESS FOR DOUBLE CARBONYLATION OF ALLYL ALCOHOLS TO CORRESPONDING DIESTERS申请人:EVONIK DEGUSSA GMBH公开号:US20170174610A1公开(公告)日:2017-06-22The invention relates to a process for doubly carbonylating allyl alcohols to the corresponding diesters, wherein a linear or branched allyl alcohol is reacted with a linear or branched alkanol (alcohol) with supply of CO and in the presence of a catalytic system composed of a palladium complex and at least one organic phosphorus ligand and in the presence of a hydrogen halide selected from HCl, HBr and HI.
表征谱图
-
氢谱1HNMR
-
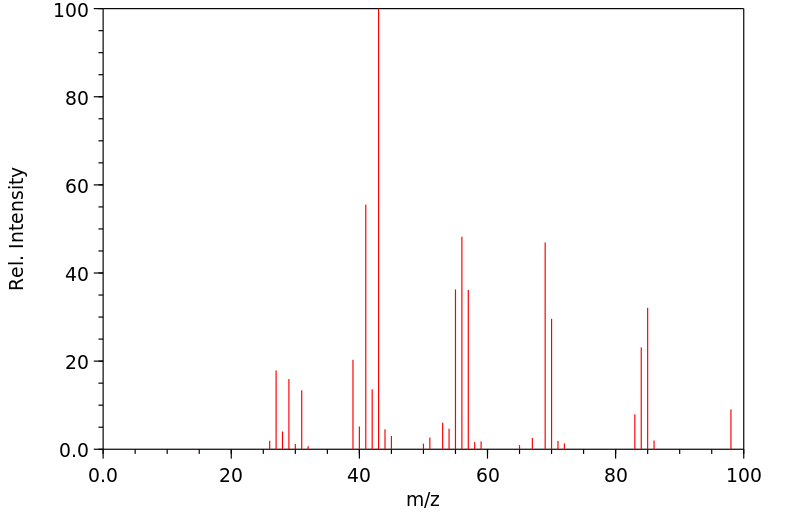
质谱MS
-
碳谱13CNMR
-
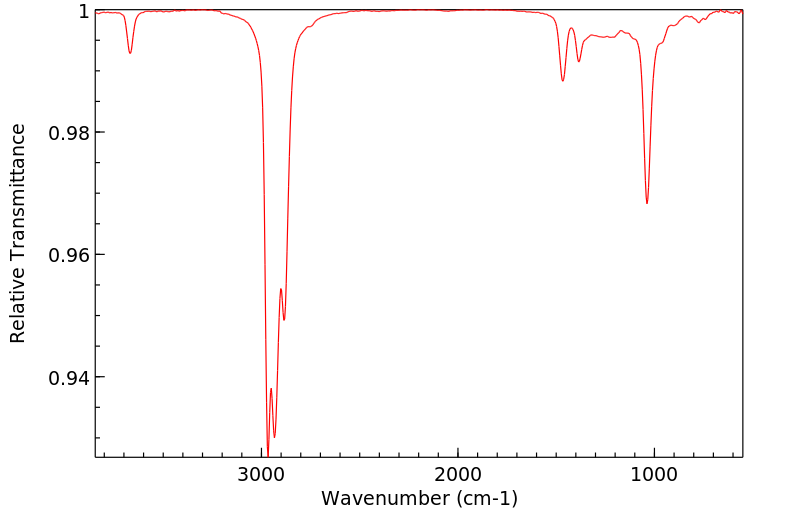
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷