N-benzyl-1H-indol-4-amine | 35245-93-3
中文名称
——
中文别名
——
英文名称
N-benzyl-1H-indol-4-amine
英文别名
4-benzylaminoindole;benzyl-indol-4-yl-amine;4-Benzylaminoindol
CAS
35245-93-3
化学式
C15H14N2
mdl
——
分子量
222.29
InChiKey
AOOQLCKCCFATCX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:431.1±20.0 °C(Predicted)
-
密度:1?+-.0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:17
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:27.8
-
氢给体数:2
-
氢受体数:1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氨基吲哚 4-amino-1H-indole 5192-23-4 C8H8N2 132.165
反应信息
-
作为反应物:描述:参考文献:名称:4-烷基氨基吲哚的意外重排摘要:在沸腾的甲苯中存在10mol%的水合甲苯-对磺酸的情况下,4-烷基氨基吲哚以良好的产率重排为相应的1-烷基-4-氨基吲哚。DOI:10.1039/c39820001356
-
作为产物:描述:参考文献:名称:4-烷基氨基吲哚的意外重排摘要:在沸腾的甲苯中存在10mol%的水合甲苯-对磺酸的情况下,4-烷基氨基吲哚以良好的产率重排为相应的1-烷基-4-氨基吲哚。DOI:10.1039/c39820001356
文献信息
-
Solvent‐Mediated C3/C7 Regioselective Switch in Chiral Phosphoric Acid‐Catalyzed Enantioselective Friedel‐Crafts Alkylation of Indoles with α‐Ketiminoesters作者:Yunlong Zhao、Lu Cai、Tongkun Huang、Shanshui Meng、Albert S. C. Chan、Junling ZhaoDOI:10.1002/adsc.201901380日期:2020.3.17The first solvent‐mediated tunable C3/C7 regio‐ and enantioselective Friedel‐Crafts alkylation of 4‐aminoindoles with α‐ketimino esters has been developed. This catalysis allows the highly regioselective formation of indole C3 and C7 alkylation products, both in high yields (up to 96%) and excellent enantioselectivities (up to 99% ee). Mechanism study revealed that the hydrogen‐bonding interactions
-
C7‐Functionalization of Indoles via Organocatalytic Enantioselective Friedel‐Crafts Alkylation of 4‐Amino‐ indoles with 2‐Butene‐1,4‐diones and 3‐Aroylacrylates作者:Tongkun Huang、Yunlong Zhao、Shanshui Meng、Albert S. C. Chan、Junling ZhaoDOI:10.1002/adsc.201900377日期:2019.8.5An efficient protocol for the enantioselective C7 Friedel‐Crafts alkylation between 4‐aminoindoles and 2‐butene‐1,4‐diones or 3‐aroylacrylates was reported. This process was catalyzed by a chiral phosphoric acid, affording the corresponding 1,4‐disubstituted indoles in moderate to high yields with good to high enantioselectivities. This reaction could be performed on a gram scale without loss of efficiency
-
Chiral Phosphoric-Acid-Catalyzed Regioselective and Enantioselective C7-Friedel–Crafts Alkylation of 4-Aminoindoles with Trifluoromethyl Ketones作者:Lu Cai、Yunlong Zhao、Tongkun Huang、Shanshui Meng、Xian Jia、Albert S. C. Chan、Junling ZhaoDOI:10.1021/acs.orglett.9b00821日期:2019.5.17trifluoromethyl ketones promoted by a spirocyclic phosphoric acid was developed. This strategy was applicable to various substituted trifluoromethyl ketones and 4-aminoindole derivatives, affording the corresponding C7-functionalized indole derivatives, bearing a pharmaceutically interesting trifluoromethylated tertiary alcohol scaffold, in 21%–98% yields with up to >99% enantiomeric excess (ee).
-
Regio and Enantioselective Organocatalytic Friedel–Crafts Alkylation of 4-Aminoindoles at the C7-Position作者:Wen Xun、Bo Xu、Bo Chen、Shanshui Meng、Albert S. C. Chan、Fayang G. Qiu、Junling ZhaoDOI:10.1021/acs.orglett.7b03703日期:2018.2.2acid catalyzed highly regio- and enantioselective Friedel–Crafts alkylation at the indole C7-position was developed via the introduction of an alkylamine moiety at the C4-position of the indole ring. The methodology is applicable to a wide range of 4-aminoindoles and β,γ-unsaturated α-ketimino esters to furnish the corresponding C7-position functionalized chiral indole derivatives in high yields with
-
Catalytic Asymmetric C-7 Friedel–Crafts Alkylation/<i>N</i>-Hemiacetalization of 4-Aminoindoles作者:Hualing He、Yang Cao、Jun Xu、Jon C. AntillaDOI:10.1021/acs.orglett.1c00699日期:2021.4.16unique catalytic asymmetric C-7 Friedel–Crafts alkylation/N-hemiacetalization cascade reaction of 4-aminoindoles with β,γ-unsaturated α-keto esters has been described. Using a chiral magnesium H8–BINOL-derived bis(phosphate) complex as catalyst, the resulting functionalized 1,7-annulated indole scaffolds are obtained in high yields (up to 98%) and with good to excellent enantioselectivities (up to
表征谱图
-
氢谱1HNMR
-
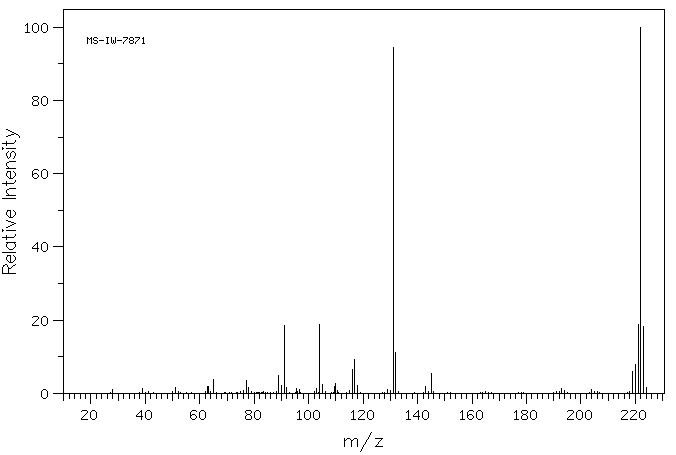
质谱MS
-
碳谱13CNMR
-
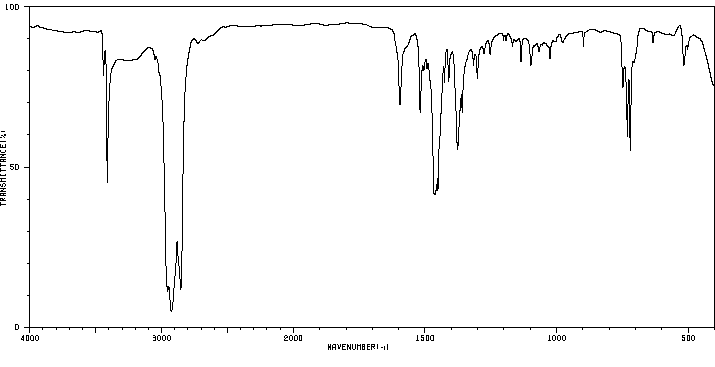
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3