3-benzyl-5-(4-methoxy-phenyl)-1-oxy-pyrazin-2-ylamine | 123437-73-0
中文名称
——
中文别名
——
英文名称
3-benzyl-5-(4-methoxy-phenyl)-1-oxy-pyrazin-2-ylamine
英文别名
——
CAS
123437-73-0
化学式
C18H17N3O2
mdl
——
分子量
307.352
InChiKey
HWHMEJZWCZUMTH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:567.8±50.0 °C(Predicted)
-
密度:1.21±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.56
-
重原子数:23.0
-
可旋转键数:4.0
-
环数:3.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:75.08
-
氢给体数:1.0
-
氢受体数:4.0
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-3-苄基-5-(4-甲氧基苯基)吡嗪 2-amino-3-benzyl-5-(4-methoxyphenyl)-pyrazine 40040-81-1 C18H17N3O 291.352 4-(5-氨基-6-苄基吡嗪-2-基)苯酚 4-(5-amino-6-benzyl-pyrazin-2-yl)-phenol 37156-84-6 C17H15N3O 277.326 —— 2-(trimethylacetylamino)-3-benzyl-5-(p-methoxyphenyl)pyrazine —— C23H25N3O2 375.47
反应信息
-
作为反应物:描述:3-benzyl-5-(4-methoxy-phenyl)-1-oxy-pyrazin-2-ylamine 在 Raney nickel W2 盐酸 、 氢气 作用下, 以 乙醇 为溶剂, 反应 96.75h, 生成 2-tert-butyl-6-(p-methoxyphenyl)-8-benzyl-3,7-dihydroimidazo<1,2-a>pyrazin-3-one参考文献:名称:腔肠萤光素荧光素类似物的低温光氧化和1,2-二氧杂环丁酮作为发光中间体的证明摘要:在2-位具有叔丁基的腔肠荧光素类似物适合在各种条件下进行化学发光研究。在低温(-78°C)下对类似物进行光氧化可得到发光中间体,该发光中间体通过用PPh 3还原而被证明是过氧化物,从而导致发光能力下降。为了通过13 C NMR阐明这些积累的发光中间体的结构,在3,7-二氢咪唑并[1,2-a]吡嗪-3-酮的2、3和5位合成了三个13 C富集的类似物。骨架具有99%的富集和位点特异性。这13个将富C的腔肠荧光素类似物在-78°C进行光氧化,以形成两种过氧化物产物,作为发光中间体。这些不稳定的中间体的结构用的手段推断13 C NMR光谱在使用在三个位点被富集的底物低的温度13 ℃。在光氧化CF的混合物3 CD 2 OD和CD 3 OD为高度的质子溶剂,得到dioxetanone和2 -氢过氧化物。在含有酸或碱的二甘醇二甲醚(DGM)中稀释到10 -5 M后,这两种过氧化物在不同的温度下分别在400DOI:10.1016/0040-4020(96)00699-0
-
作为产物:参考文献:名称:Qi, Chen Feng; Gomi, Yasushiro; Hirano, Takashi, Journal of the Chemical Society. Perkin transactions I, 1992, # 13, p. 1607 - 1612摘要:DOI:
表征谱图
-
氢谱1HNMR
-
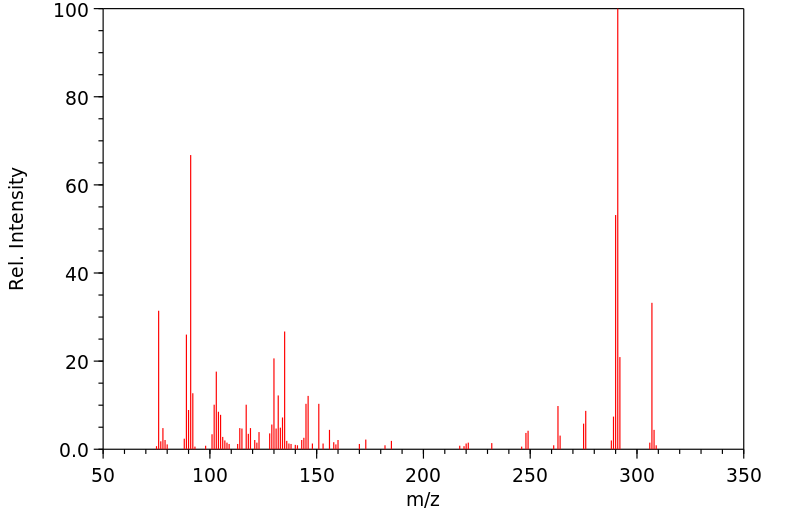
质谱MS
-
碳谱13CNMR
-
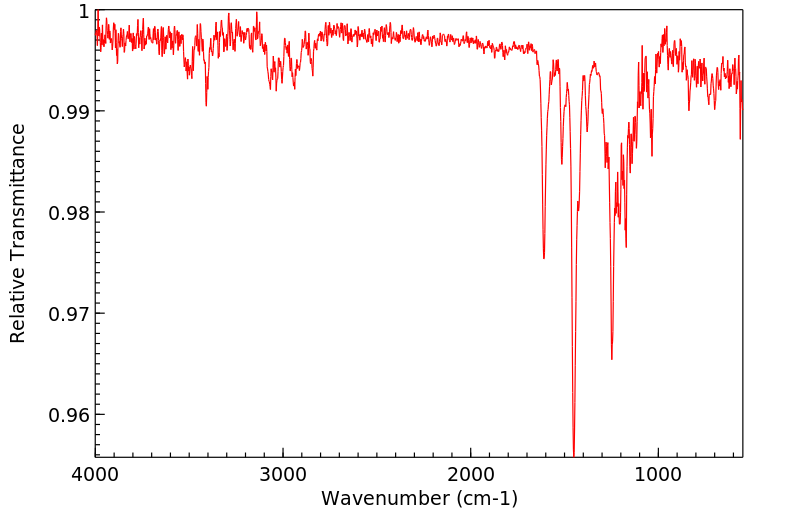
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯