2-甲基-1-氧杂螺[2.5]辛烷-2-羧酸乙酯 | 31045-09-7
中文名称
2-甲基-1-氧杂螺[2.5]辛烷-2-羧酸乙酯
中文别名
——
英文名称
ethyl 2-methyl-1-oxaspiro[2,5]octane-2-carboxylate
英文别名
2-methyl-1-oxa-spiro[2.5]octane-2-carboxylic acid ethyl ester;2-Methyl-1-oxa-spiro[2.5]octan-2-carbonsaeure-aethylester;1,α-epoxy-α-methyl-cyclohexaneacetic acid ethyl ester;ethyl 2-methyl-1-oxaspiro[2.5]octane-2-carboxylate;α-Methyl-α,β-epoxycyclohexylidenessigsaeureaethylester;2-Methyl-3,3-pentamethylen-glycidsaeure-ethylester
CAS
31045-09-7;93904-83-7
化学式
C11H18O3
mdl
——
分子量
198.262
InChiKey
DTSQPPVGQIJERI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:86 °C(Press: 0.1 Torr)
-
密度:1.0350 g/cm3
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.91
-
拓扑面积:38.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2932999099
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-(1-羟基环己基)丙酸乙酯 ethyl 2-(1-hydroxycyclohexyl)propionate 39922-60-6 C11H20O3 200.278
反应信息
-
作为反应物:描述:参考文献:名称:Darzens, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1907, vol. 144, p. 1124摘要:DOI:
-
作为产物:描述:参考文献:名称:由α,β-环氧酯合成具有全或高非对映选择性的(E)-α,β-不饱和酯。摘要:[反应:见正文]使用二碘化sa在2,3-环氧酯1中实现高立体选择性β-消除,生成α,β-不饱和酯2,其中C = C键被二,三或四取代。通过使相应的α-氯代酯的烯醇锂与醛或酮在-78℃下反应,可以轻松地制备起始化合物1。反应条件和起始化合物的结构对β-消除反应立体选择性的影响也进行了讨论。DOI:10.1021/ol016894p
文献信息
-
A highly stereoselective conversion of α,β-epoxy esters to α-hydroxy esters. An efficient route to optically active α-hydroxyesters作者:Kenji Otsubo、Junji Inanaga、Masaru YamaguchiDOI:10.1016/s0040-4039(00)96531-6日期:1987.1α,β-Epoxy esters were cleanly converted to α-hydroxy esters with retention of the configurations at the α-carbon atoms via MgI2-promoted regioselective oxirane ring-opening followed by tributyltin hydride-reduction.
-
SmI2-Induced highly regioselective reduction of α,β-epoxy esters and γ,δ-epoxy-α,β-unsaturated esters. An efficient route to optically active β-hydroxy and δ-hydroxy esters作者:Kenji Otsubo、Junji Inanaga、Masaru YamaguchiDOI:10.1016/s0040-4039(00)96532-8日期:1987.1α,β-Epoxy esters were rapidly reduced at room temperature to yield β-hydroxy esters with retention of the configurations at the β-carbon atoms by using SmI2-THF-HMPA system in the presence of N,N-dimethylaminoethanol (DMAE). The conditions were successfully applied to the synthesis of vinylogous δ-hydroxy esters.
-
Method for producing polydienes and polydiene copolymers with reduced cold flow申请人:BRIDGESTONE CORPORATION公开号:US10301397B2公开(公告)日:2019-05-28A method for preparing a coupled polymer, the method comprising the steps of (i) polymerizing monomers to form a reactive polymer, and (ii) reacting the reactive polymer with a glycidic ester.一种制备偶联聚合物的方法,该方法包括以下步骤:(i) 单体聚合形成活性聚合物,(ii) 活性聚合物与缩水甘油酯反应。
-
THE SYNTHESIS OF CERTAIN SUBSTITUTED ALICYCLIC METHYL KETONES. I作者:WILLIS AARON YARNALL、EVERETT S. WALLISDOI:10.1021/jo01215a009日期:1939.7
-
The Synthesis and Reactions of Certain α-Substituted Glycidic Esters作者:Horton H. Morris、Margaret L. LusthDOI:10.1021/ja01634a010日期:1954.3
表征谱图
-
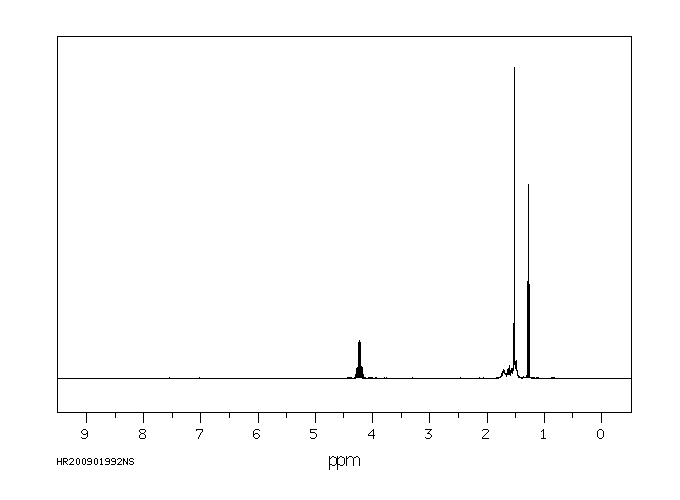
氢谱1HNMR
-
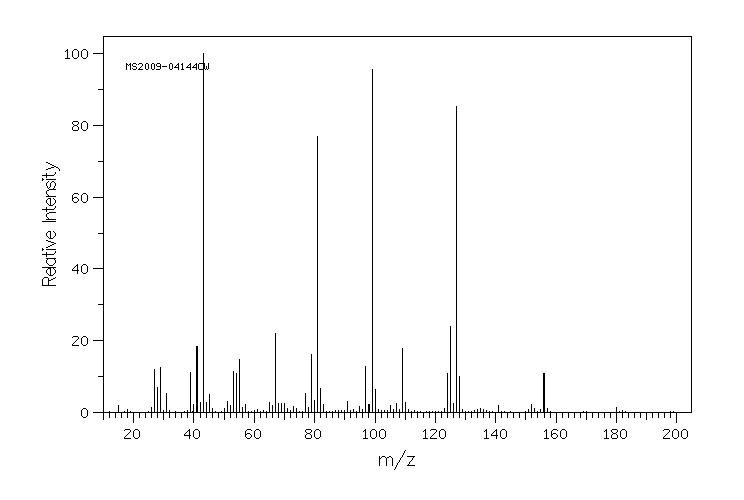
质谱MS
-
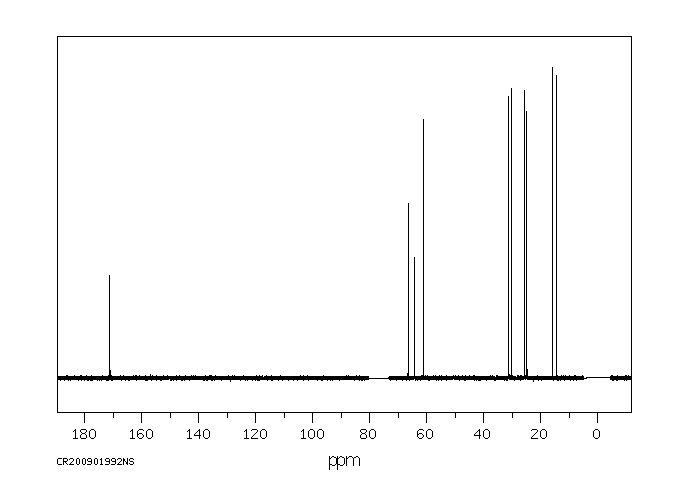
碳谱13CNMR
-
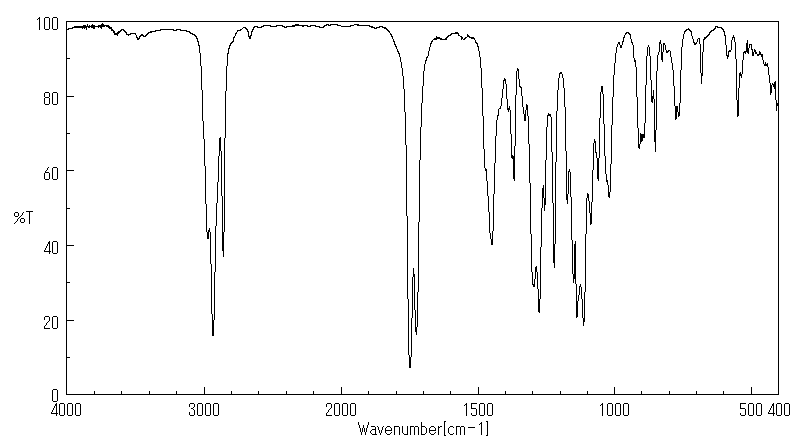
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷