2-甲基吖啶 | 613-15-0
中文名称
2-甲基吖啶
中文别名
——
英文名称
2-methylacridine
英文别名
2-Methyl-acridin
CAS
613-15-0
化学式
C14H11N
mdl
MFCD00455469
分子量
193.248
InChiKey
HVZWBVZEZZJOKQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1916.7;324.34;324.46
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Takahashi, Kazufumi; Castle, Raymond N.; Lee, Milton L., Journal of Heterocyclic Chemistry, 1987, vol. 24, p. 977 - 979摘要:DOI:
-
作为产物:参考文献:名称:Ullmann,C., Journal fur praktische Chemie (Leipzig 1954), 1887, vol. <2> 36, p. 265摘要:DOI:
文献信息
-
Cu-Catalyzed π-Core Evolution of Benzoxadiazoles with Diaryliodonium Salts for Regioselective Synthesis of Phenazine Scaffolds作者:Jinyu Sheng、Ru He、Jie Xue、Chao Wu、Juan Qiao、Chao ChenDOI:10.1021/acs.orglett.8b01748日期:2018.8.3The Cu-catalyzed regioselective synthesis of phenazine N-oxides was realized from benzoxadiazoles and diaryliodonium salts. The process was initiated by the electrophilic arylation of benzoxadiazoles with diaryliodonium salts and followed by benzocyclization reactions. The further reduction of N-oxides in situ to phenazine scaffolds and deviation to organic fluorescent materials were readily accomplished
-
Cu-Catalyzed Cascade Reaction of Isoxazoles with Diaryliodonium Salts for the Synthesis of Acridines作者:Shangrong Zhu、Xuechen Lu、Qiuneng Xu、Jian Li、Shenghu YanDOI:10.2174/1570178617666200225125427日期:2020.12.8A straightforward and efficient synthesis of acridine derivatives via a copper-catalyzed cascade reaction among isoxazoles and diaryliodonium salts is achieved. Various mono-, multi-substituted and 9-substituted acridine derivatives could be obtained in moderate to good yields. The process has gone through tandem double arylation and Friedel-Crafts reactions.
-
Radical and Ionic Mechanisms in Rearrangements of o-Tolyl Aryl Ethers and Amines Initiated by the Grubbs–Stoltz Reagent, Et3SiH/KOtBu作者:Krystian Kolodziejczak、Alexander J. Stewart、Tell Tuttle、John A. MurphyDOI:10.3390/molecules26226879日期:——investigation of the rearrangements of the aryl tolyl ethers now instead supports an anionic Truce–Smiles rearrangement, where the initial benzyl anion can be formed by either of two pathways: (i) direct deprotonation of the tolyl methyl group under basic conditions or (ii) electron transfer to an initially formed benzyl radical. By contrast, the rearrangements of o-tolyl aryl amines depend on the最近宣布了使用 Grubbs-Stoltz 试剂(Et 3 SiH + KO t Bu)重排邻甲苯基芳基醚、胺和硫化物,其中醚转化为邻羟基二芳基甲烷,而(o-tol)(Ar)NH 胺转化为二氢吖啶。根据该试剂系统中三乙基甲硅烷基自由基的先前证据,提出了自由基机制。对芳基甲苯基醚重排的详细计算研究现在支持阴离子休战-微笑重排,其中初始苄基阴离子可以通过两种途径之一形成:(i)在碱性条件下甲苯基甲基的直接去质子化或(ii) 电子转移到最初形成的苄基。相比之下,o-的重排甲苯基芳基胺取决于胺的性质。仲胺经历 NH 的去质子化,然后发生自由基重排,形成二氢吖啶,而叔胺通过自由基和/或阴离子途径形成二氢吖啶和二芳基甲烷。总的来说,这项研究强调了由 Et 3 SiH/KO t Bu 系统形成的反应中间体之间的竞争。
-
氮蒽类化合物及其合成方法和应用
-
Facile Synthesis of Unsymmetrical Acridines and Phenazines by a Rh(III)-Catalyzed Amination/Cyclization/Aromatization Cascade作者:Yajing Lian、Joshua R. Hummel、Robert G. Bergman、Jonathan A. EllmanDOI:10.1021/ja406131a日期:2013.8.28annulations of aromatic azides with aromatic imines and azobenzenes to give acridines and phenazines, respectively. These transformations proceed through a cascade process of Rh(III)-catalyzed amination followed by intramolecular electrophilic aromatic substitution and aromatization. Acridines can be directly prepared from aromatic aldehydes by in situ imine formation using catalytic benzylamine.
表征谱图
-
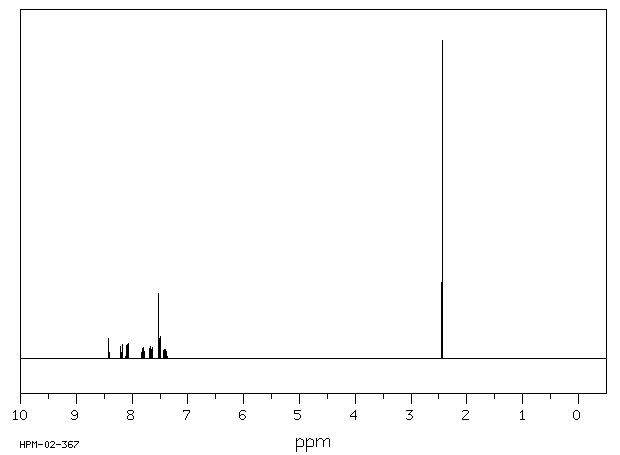
氢谱1HNMR
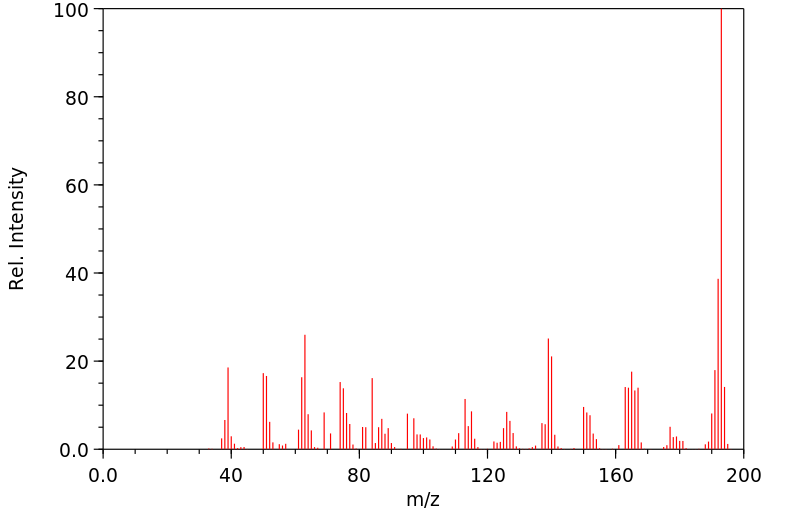
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43