2-羟基马尿酸 | 487-54-7
中文名称
2-羟基马尿酸
中文别名
O-羟基马尿酸
英文名称
Salicyluric acid
英文别名
2-hydroxyhippuric acid;2-[(2-hydroxybenzoyl)amino]acetic acid
CAS
487-54-7
化学式
C9H9NO4
mdl
——
分子量
195.175
InChiKey
ONJSZLXSECQROL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:164-169 °C
-
沸点:331.83°C (rough estimate)
-
密度:1.3544 (rough estimate)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
物理描述:Solid
-
碰撞截面:133.01 Ų [M-H]- [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
-
稳定性/保质期:
在常温常压下稳定,应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:86.6
-
氢给体数:3
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2924299090
-
RTECS号:MR8160000
-
WGK Germany:3
-
储存条件:密封保存,应储存在阴凉干燥的仓库中。
SDS
| Name: | 2-Hydroxyhippuric acid 97% Material Safety Data Sheet |
| Synonym: | Glycine, N-(2-hydroxybenzoyl)-; Glycine, N-salicyloyl-; N-(2-Hydroxybenzoyl)glycine; N-o-Hydroxybenzoylglycine; o-Hydroxyhippuric acid; Salicyloylglycine; Salicyluric acid |
| CAS: | 487-54-7 |
Synonym:Glycine, N-(2-hydroxybenzoyl)-; Glycine, N-salicyloyl-; N-(2-Hydroxybenzoyl)glycine; N-o-Hydroxybenzoylglycine; o-Hydroxyhippuric acid; Salicyloylglycine; Salicyluric acid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 487-54-7 | 2-Hydroxyhippuric acid | 97 | 207-661-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Dusts at sufficient concentrations can form explosive mixtures with air. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use extinguishing media most appropriate for the surrounding fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 487-54-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: light gray
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 167.00 - 169.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H9NO4
Molecular Weight: 195.17
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Thermal decomposition or combustion may produce carbon monoxide, carbon dioxide, and nitrogen oxides..
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 487-54-7: MR8160000 LD50/LC50:
Not available.
Carcinogenicity:
2-Hydroxyhippuric acid - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 487-54-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 487-54-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 487-54-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
生物活性方面,salicyluric acid 是一种内源性代谢产物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-(2-羟基苯甲酰基)甘氨酸甲酯 salicyluric acid methyl ester 55493-89-5 C10H11NO4 209.202 2-[(2-羟基苯甲酰基)氨基]乙酸乙酯 ethyl N-salicyloylaminoacetate 5853-89-4 C11H13NO4 223.229 龙胆酸 N-(2,5-dihydroxybenzoyl)glycine 25351-24-0 C9H9NO5 211.174 —— N-salicyloyl-glycine amide 56145-98-3 C9H10N2O3 194.19 甲基N-(2-甲氧基苯甲酰基)甘氨酸酯 (2-methoxyphenyl)(methoxycarbonylmethylamino)-methanone 27796-49-2 C11H13NO4 223.229 —— (2-Hydroxy-benzoylamino)-acetic acid benzyl ester 178633-73-3 C16H15NO4 285.299 —— 2-hydroxybenzoyl azide 54539-61-6 C7H5N3O2 163.136 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(2-羟基苯甲酰基)甘氨酸甲酯 salicyluric acid methyl ester 55493-89-5 C10H11NO4 209.202 羟基[(2-羟基苯甲酰基)氨基]乙酸 N-salicyloyl-α-hydroxyglycine 136492-94-9 C9H9NO5 211.174 —— N,N'-(2,2'-(2,2'-disulfanediylbis(ethane-2,1-diyl)bis(azanediyl))bis(2-oxoethane-2,1-diyl))bis(2-hydroxybenzamide) 1429128-83-5 C22H26N4O6S2 506.604 水杨酰胺 salicylamide 65-45-2 C7H7NO2 137.138 —— 2-hydroxy-N-(2-oxo-2-(2-oxotetrahydrothiophen-3-ylamino)ethyl)benzamide 1258323-53-3 C13H14N2O4S 294.331
反应信息
-
作为反应物:参考文献:名称:Flexible synthesis of polyamine catecholamides摘要:DOI:10.1021/jo00335a041
-
作为产物:参考文献:名称:氨基酰胆碱。2. Mitteilung。Bildung vonSalicoyl-aminosäurenaus O-(Cbzo-α-aminoacyl)-salicylsäuren摘要:水杨基水杨基中的邻苯二甲氧基邻苯二甲氧基羰基-α-氨酰基)-水杨基苯胺基苯。在Zimmertemperatur的中性染料oder schwach saurerLösung(Methylcellosolve,Eisessig)中死于Einlagerung erfolgt。DOI:10.1002/hlca.19570400644
文献信息
-
Structural effects of N-aromatic acyl-amino acid conjugates on their deconjugation in the cecal contents of rats: implication in design of a colon-specific prodrug with controlled conversion rate at the target site作者:Hyesik Kong、Hyunjeong Kim、Heejeong Do、Yonghyun Lee、Sungchae Hong、Jeong-Hyun Yoon、Yunjin Jung、Young Mi KimDOI:10.1002/bdd.763日期:2011.9N-aromatic acyl-amino acid conjugates was examined in the cecal contents. On incubation of conjugates with glycine, D or/and L forms of alanine or phenylalanine in the cecal contents, the conjugates with D amino acids were not hydrolysed. The other conjugates are susceptible to the hydrolysis, the rates of which decreased as the size of the substituent on the 2-position of the amino acids increased. The conjugatesN-芳族酰基-氨基酸缀合物具有靶向结肠的特性,这意味着此类缀合物是稳定的并且在到达大肠之前不被吸收,在大肠中它们被微生物转化(水解)为具有治疗活性的母体药物。为了研究N-芳族酰基-氨基酸缀合物对大肠去结合的结构影响,在盲肠内容物中检查了各种N-芳族酰基-氨基酸缀合物的水解。在将共轭物与甘氨酸,盲肠内容物的D或/和L形式的丙氨酸或苯丙氨酸一起孵育后,具有D氨基酸的共轭物不被水解。其他缀合物易于水解,其速率随着氨基酸2-位上取代基大小的增加而降低。具有甘氨酸和牛磺酸的烷基类似物(2-4个碳原子)的结合物具有抗水解性,而牛磺酸和甘氨酸的结合物则被有效地水解。N-芳族酰基-甘氨酸缀合物的水解通过芳族酰基部分上的吸电子基团的对位取代而得到增强,反之亦然。尽管邻位上的甲基,甲氧基或氯基阻止了水解,但位上的羟基却加速了水解。我们的数据可能为设计大肠内转化率受控的结肠特异性前药提供有用的信息。N-芳族酰基
-
[EN] INHIBITORS OF HCV NS5B POLYMERASE<br/>[FR] INHIBITEURS DE LA POLYMERASE NS5B DU VHC申请人:UPJOHN CO公开号:WO2004002977A1公开(公告)日:2004-01-08The present invention porivdes compounds of Formula I, compositons and methods that are useful for treating viral infections and associated diseases, particularly HCV infections and associated diseases.本发明提供了式I化合物、组合物及方法,这些化合物、组合物及方法对于治疗病毒感染及相关疾病具有效用,特别是HCV感染及相关疾病。
-
Synthesis of Cyclic Depsipeptides and Peptidesvia Direct Amide Cyclization作者:Jos� M. Villalgordo、Heinz HeimgartnerDOI:10.1002/hlca.19970800312日期:1997.5.12depsipeptides and peptides derived from salicylic acids 6 and anthranilic acid 19, respectively (Schemes 2--4 and 5, resp.). The combination of the ‘azirine/oxazolone method’ for the synthesis of linear peptides containing α,α-disubstituted α-amino acids and the acid-catalyzed amide cyclization in DMF at 60° proved to be an excellent preparative route to ten-membered cyclic depsipeptides and peptides. In the
-
Evaluation of aspirin metabolites as inhibitors of hypoxia-inducible factor hydroxylases作者:Benoit M. Lienard、Ana Conejo-García、Ineke Stolze、Christoph Loenarz、Neil J. Oldham、Peter J. Ratcliffe、Christopher J. SchofieldDOI:10.1039/b814440k日期:——Known and potential aspirinmetabolites were evaluated as inhibitors of oxygen-sensing hypoxia-inducible transcription factor (HIF) hydroxylases; some of the metabolites were found to stabilise HIF-α in cells.
-
Hydrophobicity and glutathione peroxidase-like activity of substituted salicyloyl-5-seleninic acids: Re-investigations on aromatic selenium compounds based on their hydrophobicity作者:Sun-Chol Yu、Dong-Myong Ri、Hartmut KühnDOI:10.1016/j.jorganchem.2018.02.045日期:2018.5salicylic acid derivatives exhibit glutathione peroxidase (GPx)-like activities higher than or equal to ebselen [Yu et al., Chem. Eur. J., 2008, 14, 7066; Org. Biomol. Chem., 2010, 8, 828]. For understanding the absence of GPx-like activity of the homologue of 5-seleninic anhydride of salicyloylglycine with a loger side chain, we have further synthesized 19 new derivatives (5-seleninic acids of methyl先前我们已经表明,一些5-硒化水杨酸衍生物表现出的谷胱甘肽过氧化物酶(GPx)样活性高于或等于依伯硒仑[Yu et al。,Chem。欧元。J.,2008,14,7066; 单位 生物分子 化学 ,2010,8,828]。为了解不存在具有loger侧链的水杨酰甘氨酸5-硒酸酐的同系物没有GPx样活性的情况,我们进一步合成了19种新衍生物(甲基或苯基水杨酸酯的5-硒酸,N-水杨酰ω-羧烷基胺或ñ-水杨酰基烷基/苯基胺,及其一些二硒化物)。某些带有长侧链或环己基的5-硒酸,无论其衍生自ω-羧烷基胺还是简单的烷基胺,都没有GPx样的活性。缺乏类似GPx的活性,使我们可以定量地将上述3个系列同源物的GPx活性与其疏水性(ClogP)相关联,在每个系列中均显示出令人满意的相关性。然后将分子疏水性广泛应用于包括二芳基二硒化物和依布硒仑衍生物在内的各种已知的芳香族硒GPx模拟物,以相对定量的方式解释其G
表征谱图
-
氢谱1HNMR
-
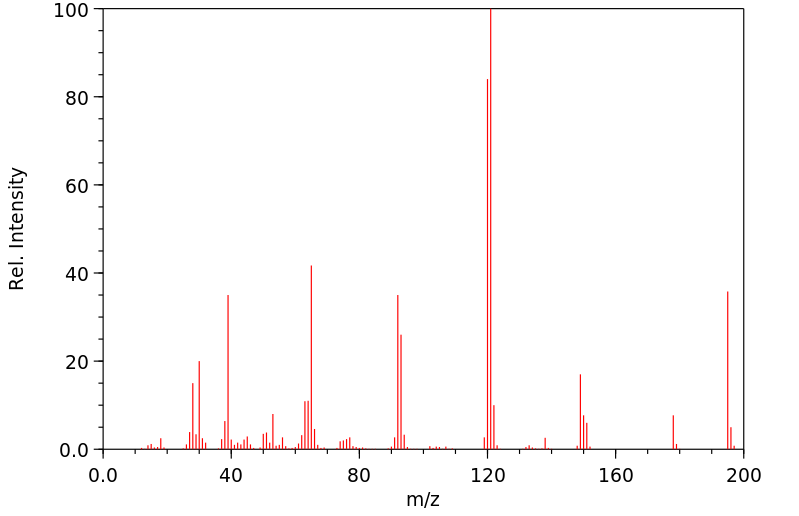
质谱MS
-
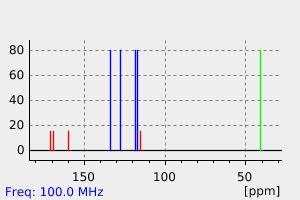
碳谱13CNMR
-
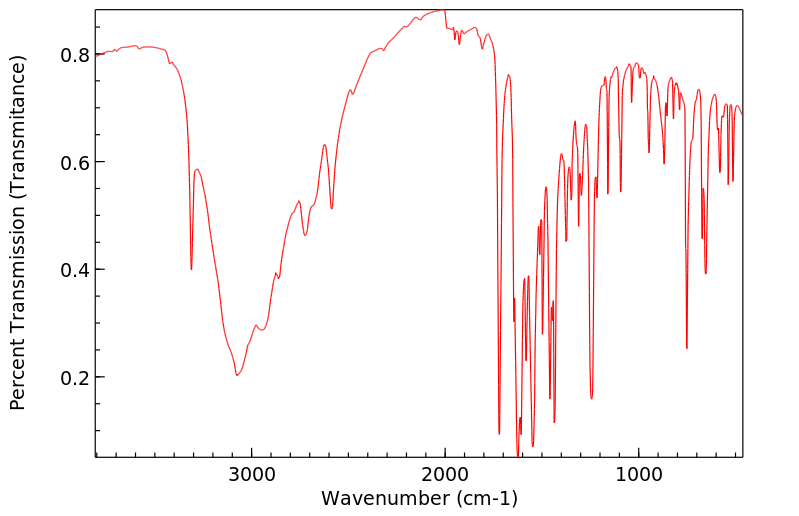
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫