2-苯基-1,2-二氢-3H-吲唑-3-酮 | 17049-65-9
中文名称
2-苯基-1,2-二氢-3H-吲唑-3-酮
中文别名
——
英文名称
2-phenyl-1,2-dihydro-3H-indazol-3-one
英文别名
2-phenyl-1,2-dihydro-indazol-3-one;2-phenyl-1,2-dihydroindazol-3-one;N2-phenylindazolone;2-phenyl-1H-indazol-3(2H)-one;2-phenyl-1H-indazol-3-one;2-phenylindazol-3-one;3-Indazolinone, 2-phenyl-
CAS
17049-65-9
化学式
C13H10N2O
mdl
——
分子量
210.235
InChiKey
CFNJFZDGHGYAMU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204 °C
-
沸点:360.8±25.0 °C(Predicted)
-
密度:1.264±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:16
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:32.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2933990090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Mosby, Chemistry and industry, 1956, p. 1524摘要:DOI:
-
作为产物:描述:2-(2-nitrophenyl)-2-phenylaminoacetonitrile 在 sodium sulfite 作用下, 生成 2-苯基-1,2-二氢-3H-吲唑-3-酮参考文献:名称:Reissert; Lemmer, Chemische Berichte, 1926, vol. 59, p. 357摘要:DOI:
文献信息
-
A Convenient Synthesis of 1-Aryl- and 2-Aryl-Substituted Indazolones via Intramolecular C–N Coupling Promoted by KOt-Bu作者:Xue-jing Zhang、Ming Yan、Wei-juan Wang、Jia-hua Chen、Zi-cong Chen、Yu-feng Zeng、Albert ChanDOI:10.1055/s-0035-1562430日期:——Abstract A new method for the synthesis of 1-arylindazolones and 2-arylindazolones from N′-aryl-2-halobenzohydrazides promoted by KOt-Bu was developed. The difference of 2-halogen substituent exerted a significant effect on the distribution of the products. Two distinct reaction pathways are proposed for the generation of 1-arylindazolones and 2-arylindazolones, respectively. A new method for the synthesis
-
一种合成吲唑酮类化合物的方法
-
Davis–Beirut Reaction: A Photochemical Brønsted Acid Catalyzed Route to <i>N</i>-Aryl 2<i>H</i>-Indazoles作者:Niklas Kraemer、Clarabella J. Li、Jie S. Zhu、Julio M. Larach、Ka Yi Tsui、Dean J. Tantillo、Makhluf J. Haddadin、Mark J. KurthDOI:10.1021/acs.orglett.9b02213日期:2019.8.2The Davis–Beirut reaction provides access to 2H-indazoles from aromatic nitro compounds. However, N-aryl targets have been traditionally challenging to access due to competitive alternate reaction pathways. Previously, the key nitroso imine intermediate was generated under alkaline conditions, but as reported here, the photochemistry of o-nitrobenzyl alcohols empowered Brønsted acid catalyzed conditions
-
Ruthenium(<scp>ii</scp>)-catalyzed synthesis of indazolone-fused cinnolines <i>via</i> C–H coupling with diazo compounds作者:Lin Su、Zheng Yu、Peiling Ren、Zhi Luo、Wei Hou、Hongtao XuDOI:10.1039/c8ob02071j日期:——for the synthesis of 12H-indazolo[2,1-a]cinnolin-12-ones was developed. Significantly, a less developed cationic complex [Ru(p-cymene)(MeCN)3(SbF6)2] was found to be effective for this transformation. In this reaction, a tandem pathway of C–H ruthenation, Ru(II)–carbene formation, migratory insertion and condensation was involved. The results of a primary mechanistic study suggested that the C–H activation
-
Palladium-Catalyzed Carbonylative Cyclization of Azoarenes作者:Zechao Wang、Zhiping Yin、Fengxiang Zhu、Yahui Li、Xiao-Feng WuDOI:10.1002/cctc.201700679日期:2017.10.10CO-n game: An efficient protocol for the palladium-catalyzed carbonylation of azobenzenes is established through C(sp2)−H bond activation with Mo(CO)6 as the solid CO source. Reactions of both symmetrical and unsymmetrical azobenzenes proceed efficiently by this procedure with high regioselectivity. BQ=p-benzoquinone.
表征谱图
-
氢谱1HNMR
-
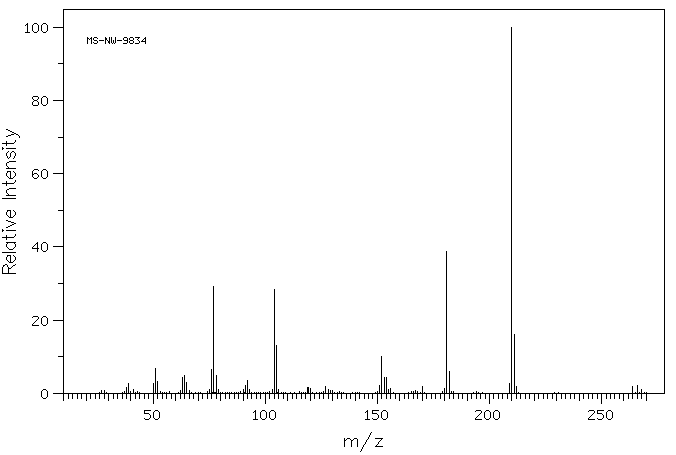
质谱MS
-
碳谱13CNMR
-
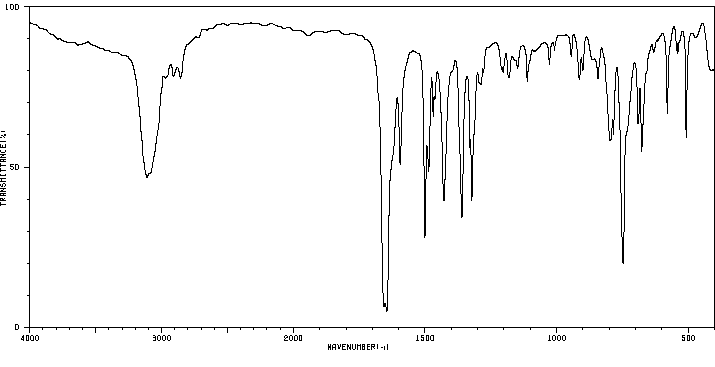
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)