3,5-二甲氧基苯基异硫氰酸酯 | 104968-58-3
中文名称
3,5-二甲氧基苯基异硫氰酸酯
中文别名
3,5-二甲氧基异硫氰酸苯酯;3,5-二甲氧基苯基硫代异氰酸酯
英文名称
1-isothiocyanato-3,5-dimethoxybenzene
英文别名
(3,5-dimethoxyphenyl)isothiocyanate;3,5-dimethoxyphenyl isothiocyanate;3,5-dimethoxy-phenyl isothiocyanate;3,5-Dimethoxy-phenylisothiocyanat;3,5-dimethoxyphenylisothiocyanate;3,5-dimethoxyisothiocyanate
CAS
104968-58-3
化学式
C9H9NO2S
mdl
MFCD00041077
分子量
195.242
InChiKey
FKUHOOASBHTEQY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:50 °C
-
沸点:130 °C
-
密度:1.12±0.1 g/cm3(Predicted)
-
闪点:130-132°C/3mm
-
溶解度:溶于氯仿
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:62.9
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:8
-
危险品标志:Xn
-
安全说明:S26,S27,S38
-
危险类别码:R20/21/22,R36/37/38
-
危险品运输编号:2811
-
海关编码:2930909090
-
包装等级:III
-
危险类别:8
-
WGK Germany:3
-
储存条件:密封储存于阴凉、干燥的库房中,远离氧化剂、水源和湿气,并常用惰性气体进行保护。
SDS
| Name: | 3 5-Dimethoxyphenyl isothiocyanate 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 104968-58-3 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 104968-58-3 | 3,5-Dimethoxyphenyl isothiocyanate | 97% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.Moisture sensitive.
Potential Health Effects
Eye:
Causes eye irritation. Lachrymator (substance which increases the flow of tears).
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Store under an inert atmosphere.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 104968-58-3: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: colorless - pale yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 130 - 132 deg C @3mmHg
Freezing/Melting Point: 50 - 52 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H9NO2S
Molecular Weight: 195
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Bases, oxidizing agents, reducing agents, amines.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 104968-58-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,5-Dimethoxyphenyl isothiocyanate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 104968-58-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 104968-58-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 104968-58-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-二甲氧基苯胺 3,5-Dimethoxyaniline 10272-07-8 C8H11NO2 153.181 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (3,5-dimethoxyphenyl)thiourea —— C9H12N2O2S 212.272
反应信息
-
作为反应物:描述:参考文献:名称:1-aryloxy-3-(substituted aminoalkylamino)-2-propanols摘要:1-芳氧基-3-(取代氨基烷基氨基)-2-丙醇及其药学上可接受的盐具有β-肾上腺素能阻滞活性,具有一定的心脏选择性,因此可用作降压、抗心绞痛、抗心律失常和心脏保护剂,并用于治疗眼内压升高,如青光眼。公开号:US04593039A1
-
作为产物:描述:参考文献:名称:具有有效抗增殖活性的微管蛋白抑制剂N,4-二芳基-1,3-噻唑-2-胺的合成和生物评价。摘要:设计并合成了一系列含有三个带有氨基接头的芳香环的N,4-二芳基-1,3-噻唑-2-胺,作为微管蛋白抑制剂,并评估了它们在三种人类癌细胞系中的抗增殖活性。大多数目标化合物均显示出适度的抗增殖活性,N-(2,4-二甲氧基苯基)-4-(4-甲氧基苯基)-1,3-噻唑-2-胺(10s)被确定为最有效的化合物。微管蛋白聚合和免疫染色实验表明,10s以类似于CA-4的方式有效抑制微管蛋白聚合并破坏微管蛋白微管动力学。此外,10s以浓度和时间依赖性方式有效地诱导了SGC-7901细胞周期阻滞在G2 / M期。分子对接结果表明10s可以结合微管蛋白的秋水仙碱结合位点。DOI:10.1371/journal.pone.0174006
文献信息
-
Guanidine-Thiourea Bifunctional Organocatalyst for the Asymmetric Henry (Nitroaldol) Reaction作者:Yoshihiro Sohtome、Yuichi Hashimoto、Kazuo NagasawaDOI:10.1002/adsc.200505148日期:2005.10Novel bifunctional catalysts having guanidine and thiourea functional groups were developed for the asymmetric Henry (nitroaldol) reaction. Various structural developments of the catalyst revealed that the compound having an octadecyl-substituted guanidine and thiourea groups linked with a chiral spacer derived from phenylalanine, i.e., 1e, efficiently promoted the Henry reaction. This bifunctional
-
Design, synthesis, and biological evaluation of novel oxadiazole- and thiazole-based histamine H3R ligands作者:Mohammad A. Khanfar、David Reiner、Stefanie Hagenow、Holger StarkDOI:10.1016/j.bmc.2018.06.028日期:2018.8Histamine H3 receptor (H3R) is largely expressed in the CNS and modulation of the H3R function can affect histamine synthesis and liberation, and modulate the release of many other neurotransmitters. Targeting H3R with antagonists/inverse agonists may have therapeutic applications in neurodegenerative disorders, gastrointestinal and inflammatory diseases. This prompted us to design and synthesize azole-based组胺H 3受体(H 3 R)在中枢神经系统中大量表达,对H 3 R功能的调节可影响组胺的合成和释放,并调节许多其他神经递质的释放。用拮抗剂/反向激动剂靶向H 3 R可能在神经退行性疾病,胃肠道和炎性疾病中具有治疗应用。这促使我们设计和合成基于吡咯的H 3R配体,即具有基于恶二唑或噻唑的核心结构。虽然恶二唑支架的配体几乎没有活性,但噻唑基配体却非常有效,并且一些在纳摩尔浓度范围内表现出结合亲和力。发现在任意基于噻唑的H 3 R配体系列中,将4-氰基苯基部分作为任意区域,对二甲苯或哌啶氨基甲酰基连接基和/或吡咯烷或哌啶碱性头部结合的配体最活跃。活性最高的配体是计算机筛选ADMET属性和药物相似性。他们符合Lipinski和Veber的规定,并表现出口服给药,血脑屏障渗透,低肝毒性以及总体良好的毒性特征的潜在活性。
-
Benzimidazole derivatives and their use as gnrh antagonists申请人:Poitout Lydie公开号:US20050049290A1公开(公告)日:2005-03-03A subject of the present application is new benzimidazole derivatives of formula in which A, Y, R 1 , R 2 , R 3 and R 4 represent different variable groups. These products have an antagonist activity of GnRH (Gonadotropin-Releasing Hormone). The invention also relates to pharmaceutical compositions containing said products and their use for the preparation of a medicament.
-
A Novel Dual Organocatalyst for the Asymmetric Pinder Reaction and a Mechanistic Proposal Consistent with the Isoinversion Effect Thereof作者:Fotini Moschona、Athena Vagena、Veroniki P. Vidali、Gerasimos RassiasDOI:10.3390/molecules26216398日期:——and enantioselectivity. To the best of our knowledge, there has been only one attempt prior to this work towards the development of a catalytic enantioselective Pinder reaction. In our approach, we designed, synthesized, and tested dual chiral organocatalysts by combining BIMAH amines, (2-(α-(alkyl)methanamine)-1H-benzimidazoles, and a Lewis acid motif, such as squaramides, ureas and thioureas. The一般而言,Pinder 反应涉及可烯醇化酸酐和醛之间的反应,该反应首先通过 Knoevenagel 反应进行,然后进行闭环过程,生成具有至少两个手性中心的内酯。这些支架经常存在于天然产物和合成生物活性分子中,因此引起了有机合成和药物化学的浓厚兴趣,特别是在控制非对映选择性和对映选择性方面。据我们所知,在这项工作之前,只有一次尝试开发催化对映选择性 Pinder 反应。在我们的方法中,我们通过结合 BIMAH 胺、(2-(α-(烷基)甲胺)-1H-苯并咪唑和路易斯酸基序,如方酰胺、脲和硫脲,设计、合成和测试了双手性有机催化剂。最佳催化剂是带有双(3,5-三氟甲基)硫脲的异丙基BIMAH衍生物,它可以从各种芳香醛中得到Pinder产物,其非对映异构体比例>98:2,对映选择性高达92 ee%。有趣的是,这种对映选择性催化过程在较高浓度下增加,并表现出同向转化效应,即相对于温度的倒“U”形依赖性
-
Discovery of 3,4-dichloro-N-(1H-indol-5-yl)benzamide: A highly potent, selective, and competitive hMAO-B inhibitor with high BBB permeability profile and neuroprotective action作者:Ahmed Elkamhawy、Hyeon Jeong Kim、Mohamed H. Elsherbeny、Sora Paik、Jong-Hyun Park、Lizaveta Gotina、Magda H. Abdellattif、Noha A. Gouda、Jungsook Cho、Kyeong Lee、Ae Nim Pae、Ki Duk Park、Eun Joo RohDOI:10.1016/j.bioorg.2021.105352日期:2021.11Parkinson’s disease (PD), there is still a vital need to develop novel selective monoamine oxidase B (MAO-B) inhibitors as promising therapeutically active candidates for PD patients. Herein, we report the design, synthesis, and full characterization of new twenty-six indole derivatives as potential human MAO-B (hMAO-B) selective inhibitors. Six compounds (2i, 3b–e, and 5) exhibited low micromolar to由于尚未发现帕金森病 (PD) 的疾病改善疗法,因此仍然迫切需要开发新型选择性单胺氧化酶 B (MAO-B) 抑制剂作为 PD 患者的有希望的治疗活性候选物。在此,我们报告了作为潜在的人类 MAO-B ( h MAO-B) 选择性抑制剂的新 26 种吲哚衍生物的设计、合成和完整表征。六种化合物(2i、3b - e和5)对h MAO-B表现出低微摩尔至纳摩尔的抑制活性;与我们最近报道的基于N-取代吲哚的先导化合物VIII ( h MAO-B IC50 = 777 nM),化合物5 (3,4-二氯-N -(1 H-吲哚-5-基)苯甲酰胺)表现出 18 倍的效力增加 (IC 50 = 42 nM)。对h MAO- A 的选择性研究揭示了化合物5的出色选择性指数(SI > 2375),与雷沙吉兰(II,一种众所周知的 MAO-B 抑制剂,SI > 50)相比增加了 47 倍。化合物5对h MA
表征谱图
-
氢谱1HNMR
-
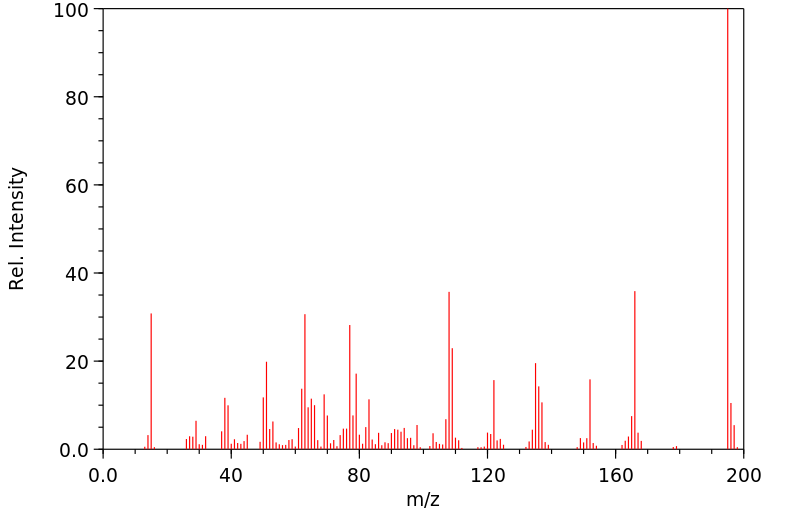
质谱MS
-
碳谱13CNMR
-
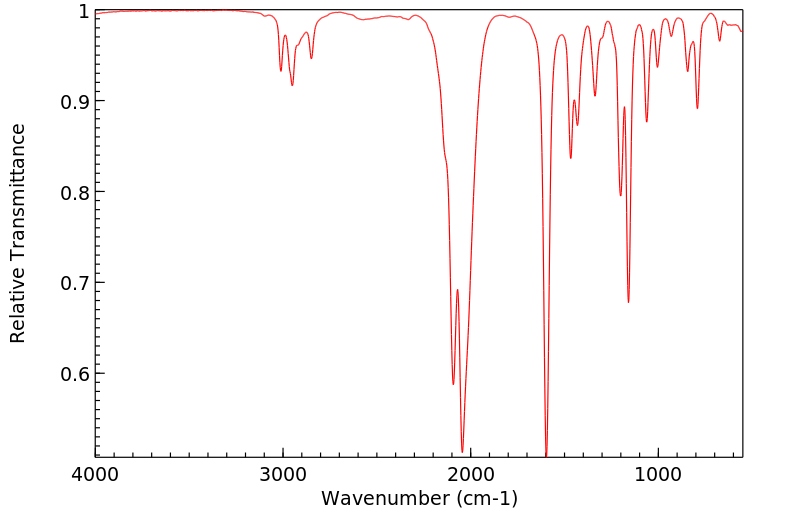
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫