3,6-二甲基-3-庚醇 | 1573-28-0
中文名称
3,6-二甲基-3-庚醇
中文别名
3-羟基-3,6-二甲基庚烷;3,6-二甲基-3-庚醇,99%
英文名称
3,6-dimethylheptane-3-ol
英文别名
3,6-dimethyl-3-heptanol;3,6-dimethylheptan-3-ol;3,6-dimethyl-heptan-3-ol;methyl-ethyl-isopentyl-carbinol;Methyl-aethyl-isopentyl-carbinol;γ-Oxy-γ.ξ-dimethyl-heptan
CAS
1573-28-0
化学式
C9H20O
mdl
——
分子量
144.257
InChiKey
HVPGGLNDHUWMLS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:6.15°C (estimate)
-
沸点:173°C
-
密度:0,82 g/cm3
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
保留指数:987;987
-
稳定性/保质期:
常温常压下稳定,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险类别码:R36/37/38
-
海关编码:2905199090
-
安全说明:S26,S36/37/39
SDS
模块 1. 化学品
1.1 产品标识符
: 3,6-Dimethyl-3-heptanol
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
严重的眼损伤 (类别1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H318 造成严重眼损伤。
警告申明
预防
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C9H20O
分子式
: 144.26 g/mol
分子量
组分 浓度或浓度范围
3,6-Dimethyl-3-heptanol
-
CAS 号 1573-28-0
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 将人员撤离到安全区域。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用惰性吸附材料吸收并当作危险废品处理。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
防止吸入蒸汽和烟雾。
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
紧密装配的防护眼镜请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 3.069
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
长期或反复接触导致个别人过敏反应
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 引起眼睛烧伤。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Halse, Journal fur praktische Chemie (Leipzig 1954), 1914, vol. <2>89, p. 452摘要:DOI:
-
作为产物:描述:参考文献:名称:Abbot,E.M.; Bellamy,A.J., Journal of the Chemical Society. Perkin transactions II, 1978, p. 254 - 258摘要:DOI:
文献信息
-
Regioselective Synthesis of a Branched Isomer of Nonylphenol, 4-(3′,6′-Dimethyl-3′-heptyl)phenol, and Determination of its Important Environmental Properties作者:Joseph O. Lalah、Karl-W. Schramm、Dieter Lenoir、Bernhard Henkelmann、Norbert Hertkorn、Georg Matuschek、Antonius Kettrup、Klaus GüntherDOI:10.1002/1521-3765(20011119)7:22<4790::aid-chem4790>3.0.co;2-l日期:2001.11.19A method for the synthesis of a pure nonylphenol isomer, 4-(3',6'-dimethyl-3-heptyl)phenol, by Friedel-Crafts reaction between anisole and 3-bromo-3,6-dimethylheptane that gives a 47.3% overall yield is reported. The reactions were followed by GC-MS, and the chemical structures are in agreement with the NMR and IR spectra. The log K(ow) value for this compound, its water solubility, vapor pressure
-
Synthesis of tertiary<sup>14</sup>C-labelled nonylphenol isomers作者:Ralph Vinken、Burkhard Schmidt、Andreas SchäfferDOI:10.1002/jlcr.641日期:2002.12The ring-14C-labelled p-nonylphenol (NP) isomers 4(3′,5′-dimethyl-3′-heptyl)-phenol (p353NP), 4(3′,6′-dimethyl-3′-heptyl)-phenol (p363NP) and 4(2′,6′-dimethyl-2′-heptyl)-phenol (p262NP) were synthesized for application in metabolism and sorption studies. Friedel–Crafts alkylation of 14C-labelled phenol and the corresponding tertiary nonylalcohol with BF3 as catalyst was used. After clean-up of p262NP and p363NP by preparative thin-layer chromatography radiochemical yields amounted to 62.8 and 64.6%, specific radioactivities were 332 and 88.2 MBq/mmol, and radiochemical purities 97.6 and 99.0%. For both isomers, a large-scale synthesis with non-labelled phenol was additionally developed, which led to pure products (96 and 99%, respectively) without further purification steps. In the case of p353NP, which was formed as a diastereomeric mixture, the crude synthetic product had a radiochemical purity of 96.9% (radiochemical yield: 76.0%; specific activity: 298 MBq/mmol); thus, purification was not necessary. All products were characterized by means of gas chromatography-mass spectroscopy, 1H- and 13C-NMR, as well as IR. Copyright © 2002 John Wiley & Sons, Ltd.三种带有14C标记的对壬基酚(NP)异构体被合成,用于代谢和吸附研究: - 4(3',5'-二甲基-3'-庚基)-酚(p353NP) - 4(3',6'-二甲基-3'-庚基)-酚(p363NP) - 4(2',6'-二甲基-2'-庚基)-酚(p262NP) 合成方法是使用BF3作为催化剂,对14C标记的酚和相应的叔壬醇进行傅-克烷基化反应。 通过制备薄层色谱纯化后: - p262NP放射化学产率为62.8%,比活度332 MBq/mmol,放射化学纯度97.6% - p363NP放射化学产率为64.6%,比活度88.2 MBq/mmol,放射化学纯度99.0% 另外还开发了这两种异构体使用非标记酚的大规模合成方法,无需进一步纯化即可得到纯度分别为96%和99%的产品。 p353NP作为非对映异构体混合物生成,粗产品的放射化学纯度为96.9%(放射化学产率76.0%,比活度298 MBq/mmol),因此无需纯化。 所有产物均通过气相色谱-质谱、1H-NMR、13C-NMR和IR进行表征。
-
Estrogen equivalent concentration of 13 branched para-nonylphenols in three technical mixtures by isomer-specific determination using their synthetic standards in SIM mode with GC–MS and two new diasteromeric isomers作者:Takao Katase、Keiji Okuda、Yun-Seok Kim、Heesoo Eun、Hideshige Takada、Taketo Uchiyama、Hiroaki Saito、Mitsuko Makino、Yasuo FujimotoDOI:10.1016/j.chemosphere.2007.09.049日期:2008.29+/-0.3% to 3.0+/-0.2%. Additionally, structures of four new isomers of more than 1% portion present in a technical mixture were elucidated as two pairs of diastereomeric isomers: two types of 4-(3,4-dimethylheptan-4-yl)phenol (344NP) and those of 4-(3,4-dimethylheptan-3-yl)phenol (343NP). By estrogenic assay of 13 isomers with yeast estrogen screen system, the activity of 3E22NP was the highest, while通过SIM的结构特异性离子m / z 135、149或163的合成标准品,使用GC-MS,通过合成标准物,在三种技术混合物中选择了13种支链对壬基酚(对-NP)异构体。在13种异构体中,有4种异构体,分别是4-(2,4-二甲基庚基-4-基)苯酚,4-(4-甲基辛基-4-基)苯酚,4-(3-乙基-2-甲基己-2-基)首先将用于测定的)苯酚(3E22NP)和4-(2,3-二甲基庚基-2-基)苯酚用作标准物质。工业混合物中的13种异构体的质量百分比含量分别超过2%。东京化学工业公司(TCI),Aldrich和Fluka的混合物中的总质量百分比分别为89 +/- 2%,75 +/- 4%和77 +/- 2%。三种混合物中4-(3,6-二甲基庚基-3-基)苯酚的丰度最大,为11.1 +/- 2%至9。9 +/- 0.3%,而4-(2-甲基辛烷-2-基)苯酚最小,为2.9 +/- 0.3%至3.0 +/-
-
Method for obtaining ethanol申请人:3M Innovative Properties Company公开号:US20040181101A1公开(公告)日:2004-09-16A method for obtaining ethanol from a feed solution utilizes an extractant comprising a mixture of at least one alkane and at least one aliphatic alcohol having the formula 1 wherein R 1 , R 2 , R 3 , R 4 , R 5 , and R 7 each independently represent H or a straight chain alkyl group having from 1 to 4 carbon atoms; R 6 represents a straight chain alkyl group having from 1 to 4 carbon atoms, or taken together R 3 and R 6 represent an alkylene group having from 1 to 4 carbon atoms; and m and n independently represent 0, 1, 2, or 3, with the proviso that if R 4 represents H, then at least two of R 1 , R 2 , and R 3 , or at least one of R 5 and R 7 , are straight chain alkyl groups having from 1 to 4 carbon atoms.
-
Funktionalisierte Prozessöle, Verfahren zu ihrer Herstellung und ihre Verwendung in Kautschukmischungen申请人:Klaus Dahleke KG公开号:EP2361949A1公开(公告)日:2011-08-31Gegenstand der Erfindung ist ein funktionalisiertes Prozessöl auf Basis von Prozessölen aus der Mineralölraffination mit verbesserten Anwendungseigenschaften, ein Verfahren zur Herstellung und die Verwendung der Prozessöle. Die erfindungsgemäßen Prozessöle können u.a. als Weichmacher in Kautschuken oder Kautschukprodukten eingesetzt werden und führen zu einem verbesserten Verarbeitungs- und Alterungsverhalten.本发明的主题是一种基于矿物油精炼加工油的功能化加工油,具有更好的应用特性,以及生产和使用加工油的工艺。除其他外,本发明的加工油可用作橡胶或橡胶制品的增塑剂,从而改善加工和老化性能。
表征谱图
-
氢谱1HNMR
-
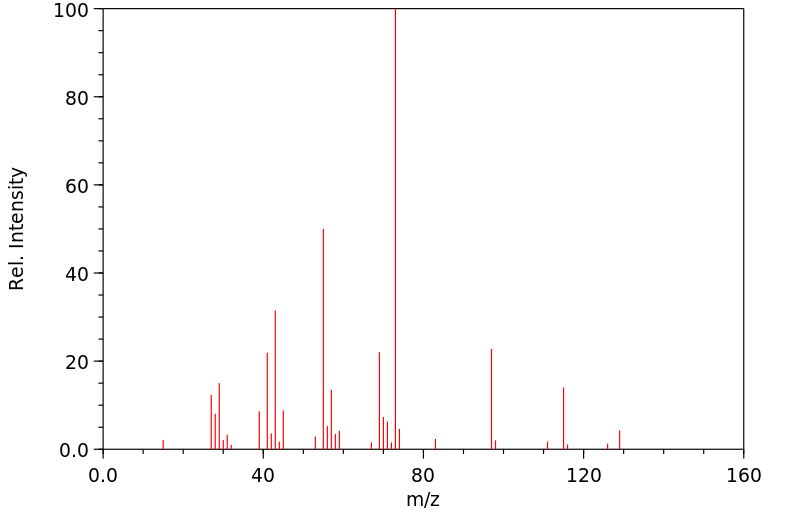
质谱MS
-
碳谱13CNMR
-
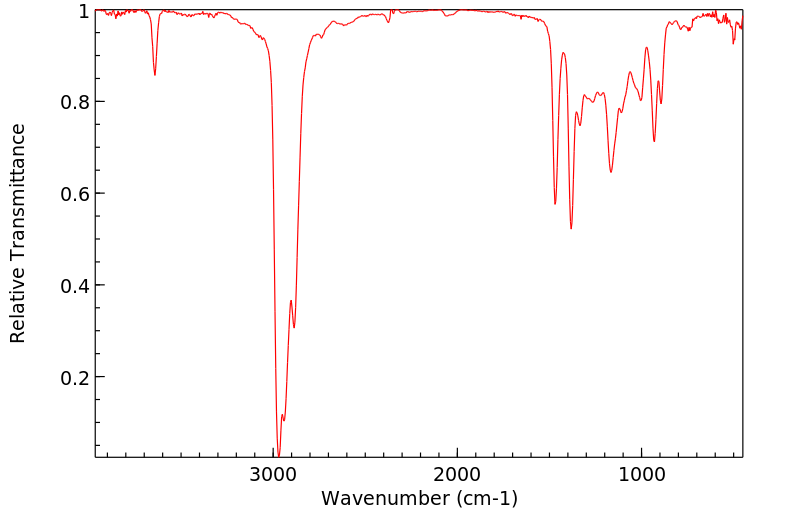
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷