2,4,6-trifluorobiphenyl | 67277-36-5
中文名称
——
中文别名
——
英文名称
2,4,6-trifluorobiphenyl
英文别名
1,3,5-trifluoro-2-phenylbenzene
CAS
67277-36-5
化学式
C12H7F3
mdl
——
分子量
208.183
InChiKey
XNAKKTLRTNNKAS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:2,4,6-trifluorobiphenyl 在 一氯化碘 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 、 N-甲基吡咯烷酮 、 二氯甲烷 为溶剂, 反应 16.0h, 生成 2,4,6-Trifluoro-5-phenylbenzene-1,3-dicarbonitrile参考文献:名称:COMPOUND, MATERIAL FOR ORGANIC ELECTROLUMINESCENT ELEMENT, ORGANIC ELECTROLUMINESCENT ELEMENT, AND ELECTRONIC DEVICE摘要:A compound is represented by a formula (1) below. D is a group represented by a formula (11), (12) or (13) below; at least one D is a group represented by the formula (12) or (13); at least one R is a substituent; and a sum of the number of R serving as a substituent and the number of a group represented by the formula (12) or (13) is 3 or 4.公开号:US20220388991A1
-
作为产物:描述:2,4,6-三氟苯甲酸 在 (1,5-环辛二烯)二(三甲基硅烷基甲基)钯(II) 、 sodium t-butanolate 、 2-二环己基磷-2,4,6-三异丙基联苯 作用下, 以 1,4-二氧六环 、 甲醇 为溶剂, 反应 26.0h, 生成 2,4,6-trifluorobiphenyl参考文献:名称:无添加剂钯催化的芳基氯化物的脱羧交叉偶联摘要:(杂)芳基羧酸钠与(杂)芳基氯化物的交叉偶联用1mol%的钯催化剂进行,不需要无机碱,银盐或铜盐。这种耦合使用两个低能伙伴,唯一的化学计量副产物是二氧化碳和氯化钠。底物范围包括较少活化的芳基氯化物和羧酸盐(> 25个实例)。钯的负载量可以降低到0.1mol%,并且可以使用布赫瓦尔德(Buchwald)型预催化剂。DOI:10.1021/acs.orglett.9b01620
文献信息
-
Base-free nickel-catalysed decarbonylative Suzuki–Miyaura coupling of acid fluorides作者:Christian A. Malapit、James R. Bour、Conor E. Brigham、Melanie S. SanfordDOI:10.1038/s41586-018-0628-7日期:2018.11Suzuki–Miyaura-type reactions to proceed without an exogenous base12–14. Here we use this approach to develop a nickel-catalysed coupling of aryl boronic acids with acid fluorides15–17, which are formed in situ from readily available carboxylic acids18–22. This combination of catalyst and electrophile enables a mechanistic manifold in which a ‘transmetallation-active’ aryl nickel fluoride intermediate is generated有机硼亲核试剂与芳基卤化物亲电子试剂的 Suzuki–Miyaura 交叉偶联是有机化学和药物化学中应用最广泛的碳-碳键形成反应之一 1,2。与这些转化相关的一个关键挑战是它们通常需要添加外源碱,其作用是使有机硼亲核试剂和金属催化剂之间发生金属转移3。这一要求限制了反应的底物范围,因为添加的碱会促进许多有机硼底物的竞争性分解 3-5。因此,相当多的研究集中在减轻碱基介导的副反应的策略上 6-12。以前的努力主要集中在设计战略性掩蔽的有机硼试剂(以减缓碱介导的分解)6-8 或开发高活性钯预催化剂(以加速与碱介导的分解途径相关的交叉偶联)10-12。一种有吸引力的替代方法涉及确定催化剂和亲电子试剂的组合,使铃木-宫浦型反应能够在没有外源碱基的情况下进行 12-14。在这里,我们使用这种方法来开发镍催化的芳基硼酸与酰基氟的偶联 15-17,后者由容易获得的羧酸原位形成 18-22。催化剂和亲电子
-
Combined Experimental and Computational Mechanistic Investigation of the Palladium-Catalyzed Decarboxylative Cross-Coupling of Sodium Benzoates with Chloroarenes作者:Jenna N. Humke、Ryan A. Daley、Aaron S. Morrenzin、Sharon R. Neufeldt、Joseph J. TopczewskiDOI:10.1021/acs.joc.1c00910日期:2021.9.3is a mechanistic investigation into the palladium-catalyzed decarboxylative cross-coupling of sodium benzoates and chloroarenes. The reaction was found to be first-order in Pd. A minimal substituent effect was observed with respect to chloroarene, and the reaction was zero-order with respect to chloroarene. Palladium-mediated decarboxylation was assigned as the turnover-limiting step based on an Eyring本文报道了对钯催化的苯甲酸钠和氯芳烃的脱羧交叉偶联的机理研究。发现反应在 Pd 中是一级反应。观察到对氯芳烃的取代作用最小,对氯芳烃反应为零级。基于艾林图和密度泛函理论计算,钯介导的脱羧被指定为转换限制步骤。发现催化剂性能因亲电试剂而异,这最好通过催化剂在 Pd(0) 处分解来解释。的1,5-环辛二烯(COD)的配体包含在前段催化剂CODPd(CH 2 TMS)2(PD1)被证明是有益的添加剂。长凳稳定的 Buchwald 复合体XPhosPdG2可以与外源COD和2-二环己基膦基-2',4',6'-三异丙基联苯(XPhos)代替复杂的使用PD1。添加外源 XPhos 显着增加了催化剂周转数并增强了重现性。
-
Copper‐Catalysed Suzuki‐Miyaura Cross‐Coupling of Highly Fluorinated Aryl Boronate Esters with Aryl Iodides and Bromides and Fluoroarene−Arene π‐Stacking Interactions in the Products作者:Yudha P. Budiman、Alexandra Friedrich、Udo Radius、Todd B. MarderDOI:10.1002/cctc.201901220日期:2019.11.7combination of copper iodide and phenanthroline as the ligand is an efficient catalyst for Suzuki‐Miyaura cross‐coupling of highly fluorinated boronate esters (aryl−Bpin) with aryl iodides and bromides to generate fluorinated biaryls in good to excellent yields. This method represents a nice alternative to traditional cross‐coupling methods which require palladium catalysts and stoichiometric amounts of
-
Palladium-Catalyzed Direct Arylation of Electron-Deficient Polyfluoroarenes with Arylboronic Acids作者:Ye Wei、Jian Kan、Min Wang、Weiping Su、Maochun HongDOI:10.1021/ol901200g日期:2009.8.6The palladium-catalyzed direct arylation of electron-deficient arenes that contain two or more fluorine groups with arylboronic acids was realized. The key to achieving a broad substrate scope with respect to both polyfluorobenzenes and arylboronic acids is the choice of bases depending on acidities of polyfluorobenzenes.
-
<i>t</i>Bu<sub>3</sub>P-Coordinated 2-Phenylaniline-Based Palladacycle Complexes as Precatalyst for Pd-Catalyzed Coupling Reactions of Aryl Halides with Polyfluoroarenes by a C-H Activation Strategy作者:Hong-Hai Zhang、Jie Dong、Qiao-Sheng HuDOI:10.1002/ejoc.201301272日期:2014.2tBu3P-Coordinated 2-phenylaniline-based palladacycle complex was demonstrated to be an efficient precatalyst for Pd-catalyzed coupling reactions of aryl halides with polyfluoroarenes via C-H activation strategy. The readily accessibility and easy handling nature of tBu3P- coordinated 2-aminobiphenyl-based palladacycle complex and the high yields of the reaction makes tBu3P-coordinated 2-aminobiphenyl-based
表征谱图
-
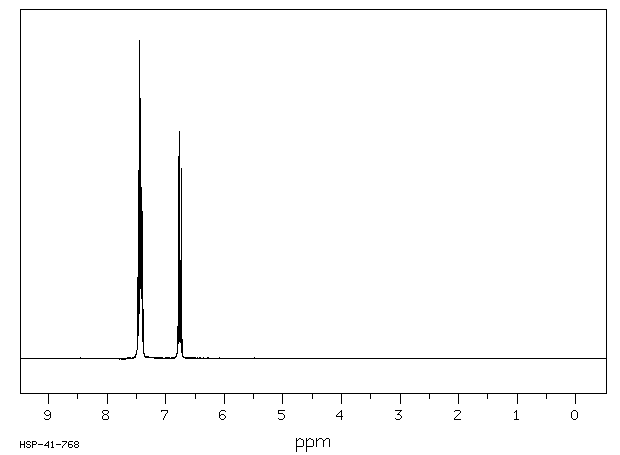
氢谱1HNMR
-
质谱MS
-
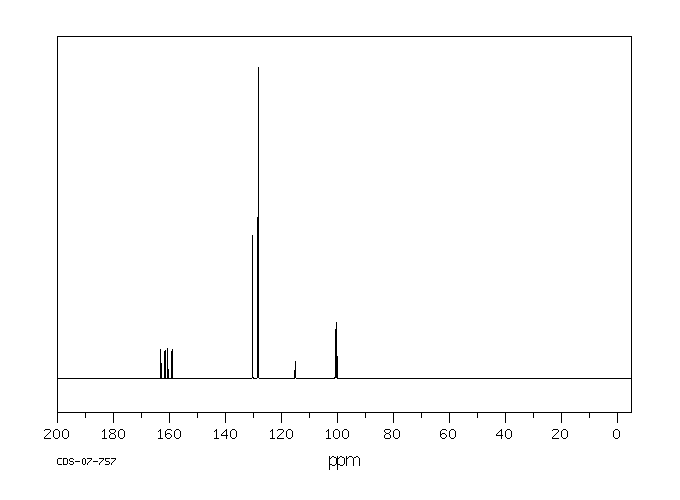
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫