3-(4-甲基苯基)-1,3-恶唑烷-2-酮 | 5198-46-9
中文名称
3-(4-甲基苯基)-1,3-恶唑烷-2-酮
中文别名
——
英文名称
3-(4-methylphenyl)-2-oxazolidinone
英文别名
3-(p-tolyl)oxazolidin-2-one;3-(4-methylphenyl)-oxazolidin-2-one;N-(4-methylphenyl)oxazolidin-2-one;2-Oxo-3-(p-tolyl)-1,3-oxazolidine;3-(4-methylphenyl)-1,3-oxazolidin-2-one
CAS
5198-46-9
化学式
C10H11NO2
mdl
——
分子量
177.203
InChiKey
CGDOGUUOMDFHLT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:91 °C
-
沸点:287.9±10.0 °C(Predicted)
-
密度:1.199±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-(p-Tolyl)carbamidsaeure-β-chlorethylester 74552-28-6 C10H12ClNO2 213.664 叔-丁基 p-苯甲基氨基甲酸酯 N-(tert-butoxycarbonyl)-4-methylaniline 14618-59-8 C12H17NO2 207.272
反应信息
-
作为反应物:描述:参考文献:名称:由2-恶唑烷酮与氰化物离子开环合成3-氨基丙腈的新方法摘要:在18-冠-6存在下,氰离子对2-恶唑烷酮的5-位的亲核攻击产生了3-氨基丙腈。DOI:10.1016/j.tetlet.2009.06.014
-
作为产物:描述:参考文献:名称:[EN] METHODS FOR PREPARING BENZOXAZINES
[FR] PROCÉDÉS DE PRÉPARATION DE BENZOXAZINES摘要:提供一种制备化合物的方法,该方法包括执行以下反应:公式(i);公式(ii)其中:R10是氢或羟基保护基;PG是胺保护基;q为1,其中PG是一价保护基,或q为0,其中PG是二价保护基;其中化合物d中的任何-XH或-NH基可能以烷酰化形式存在。公开号:WO2020260417A1
文献信息
-
Polymer-Supported Copper Complex for C−N and C−O Cross-Coupling Reactions with Aryl Boronic Acids作者:Gary C. H. Chiang、Thomas OlssonDOI:10.1021/ol048943e日期:2004.9.1[reaction: see text] Immobilization of copper onto modified Wang resin provided a polymer-supported copper catalyst, which is effective in cross-coupling reactions between N- or O-containing substrates and arylboronic acids. The copper catalyst is air stable and can be recycled with minimal loss of activity.
-
Ligand-Enabled Gold-Catalyzed C(sp<sup>2</sup>)–N Cross-Coupling Reactions of Aryl Iodides with Amines作者:Manjur O. Akram、Avishek Das、Indradweep Chakrabarty、Nitin T. PatilDOI:10.1021/acs.orglett.9b03082日期:2019.10.4example of ancillary (P,N)-ligand-enabled gold-catalyzed C-N cross-coupling reactions of aryl iodides with amines is reported. The high generality of the reaction in de novo synthesis, late-stage modifications, and cascade processes to access functionalized indolinones and carbazoles underscores the synthetic potential of the presented strategy. Monitoring the reaction with ESI-HRMS and NMR provided strong
-
2-Alkynyl-and 2-alkenyl-pyrazolo-[4,3-e]-1,2,4-triazolo-[1,5-c]-pyrimidine adenosine A2a receptor antagonists申请人:Schering Corporation公开号:US20040220194A1公开(公告)日:2004-11-04Compounds having the structural formula I 1 or a pharmaceutically acceptable salt thereof, wherein R is 2 R 1 , R 2 , R 3 , R 4 and R 5 are H, alkyl or alkoxyalkyl; R 6 is H, alkyl, hydroxyalkyl or —CH 2 F; R 7 , R 8 and R 9 are H, alkyl, alkoxy, alkylthio, alkoxyalkyl, halo or —CF 3 ; and Z is optionally substituted aryl, heteroaryl or heteroaryl-alkyl are disclosed. Also disclosed is the use of compounds of formula I in the treatment of central nervous system diseases, in particular Parkinson's disease, alone or in combination with other agents for treating Parkinson's disease, and pharmaceutical compositions comprising them.
-
Synthesis of Oxazolidinones and Derivatives through Three-Component Fixation of Carbon Dioxide作者:Congmin Mei、Yibo Zhao、Qianwei Chen、Changsheng Cao、Guangsheng Pang、Yanhui ShiDOI:10.1002/cctc.201800142日期:2018.7.19three‐component fixation of atmospheric CO2 with readily available 1,2‐dichloroethane and aromatic amine toward oxazolidinones catalyzed by in situ NHC was developed. The reaction occurred in good to excellent yields with good generality and wide functional group tolerance, including challenging steric hindered substituted‐dichloroethane (e.g. 1,2‐dichloropropane and 2,3‐dichlorobutane). The catalytic system已经开发了一种有效的三组分固定方法,用现成的NHC催化的1,2-二氯乙烷和芳香族胺直接固定在恶唑烷酮上,以固定大气中的CO 2。该反应以良好的通用性和宽泛的官能团耐受性(包括具有挑战性的位阻取代的二氯乙烷(例如1,2-二氯丙烷和2,3-二氯丁烷))以良好的收率获得了良好的收率。该催化体系对空气和湿气不敏感,即使在约0.3atm的二氧化碳压力下也可获得高产率的恶唑烷酮。机理研究表明,该反应是通过NHC-CO 2原位催化NHC催化的加合物中,1-丁基-3-甲基咪唑-2-羧酸盐为中间体,而氨基氯化物参与反应路径。催化剂可以循环使用至少3次而不会明显降低催化活性。该协议还可以有效地制备六元3-芳基-1,3-氧杂嗪酮-2-一,甚至七元3-芳基-1,3-氧杂氮杂-2-酮,当1,3-二氯丙烷和1时使用了4-二氯丁烷。
-
Copper-Catalyzed One-Pot Synthesis of <i>N</i>-Aryl Oxazolidinones from Amino Alcohol Carbamates作者:William Mahy、Pawel K. Plucinski、Christopher G. FrostDOI:10.1021/ol502322c日期:2014.10.3sequential intramolecular cyclization of amino alcohol carbamates followed by Cu-catalyzed cross-coupling with aryl iodides under mild conditions has been developed. The reaction occurred in good yields and tolerated aryl iodides containing functionalities such as nitriles, ketones, ethers, and halogens. Heteroaryl iodides and substituted amino alcohol carbamates were also well tolerated.
表征谱图
-
氢谱1HNMR
-
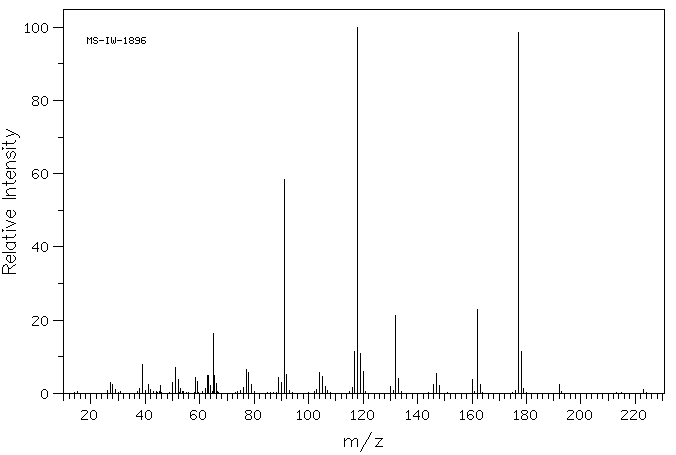
质谱MS
-
碳谱13CNMR
-
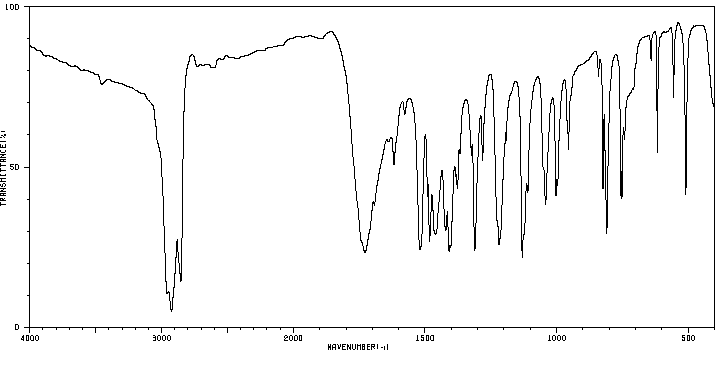
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫