甲草胺 | 15972-60-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:39-42°C
-
沸点:100°C (0.02 mmHg)
-
密度:d2515.6 1.133
-
闪点:-18 °C
-
溶解度:DMF:30mg/mL,DMSO:30mg/mL
-
物理描述:Alachlor is a crystalline solid. Melting point 104-106°F (40-41°C). Used as a herbicide.
-
颜色/状态:Colorless to white crystalline solid
-
气味:Odorless
-
蒸汽密度:Relative vapor density (air = 1): 9.3
-
蒸汽压力:2.20X10-5 mm Hg at 25 °C
-
亨利常数:8.32e-09 atm-m3/mole
-
稳定性/保质期:
INDEFINITELY STABLE; NOT SENSITIVE TO LIGHT OR HEAT
-
分解:When heated to decomposition it emits very toxic fumes of /hydrogen chloride and nitrogen oxides/.
-
腐蚀性:NO CORROSION TO NUMBER 316 & 304 STAINLESS STEEL, ALUMINUM & HERESITE; CORROSIVE TO STEEL & BLACK IRON
-
碰撞截面:156.41 Ų [M+H]+; 170.33 Ų [M+Na]+
-
保留指数:1876;1897;1882;1886.4;1906.7;1889.6;1880.1;1883;1879.4;1876.7;1881.6
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:18
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
危险等级:9
-
危险品标志:Xn
-
安全说明:S36/37,S45,S46,S60,S61,S62
-
危险类别码:R22,R40,R50/53,R43
-
WGK Germany:2,3
-
危险品运输编号:UN 3077
-
RTECS号:AE1225000
-
海关编码:2924299034
-
包装等级:I; II; III
-
危险类别:6.1
-
储存条件:密封保存。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 甲草胺;2-氯-2′,6′-二乙基-N-(甲氧甲基)乙酰苯胺 |
| 化学品英文名称: | Alachlor;2-Chloro-2′,6′-diethyl-N-(methoxymethyl)acetanilide |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 15972-60-8 |
| 分子式: | C 14 H 20 ClNO 2 |
| 分子量: | 269.80 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:甲草胺;2-氯-2′,6′-二乙基-N-(甲氧甲基)乙酰苯胺 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | 第6.1类 毒害品 |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 本品为低毒除草剂。吸入、摄入或经皮肤吸收后会中毒。有刺激作用。资料报道,对人有致突变作用。受热分解释出有毒的氯气和氮氧化物。 |
| 环境危害: | 对环境有危害。 |
| 燃爆危险: | 本品可燃,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。受高热分解,放出有毒的烟气。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳、氮氧化物、氯化氢。 |
| 灭火方法及灭火剂: | 在安全距离以外,在上风向灭火。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | 消防人员须戴好防毒面具 |
| 禁止使用的灭火剂: | |
| 闪点(℃): | |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好防毒面具,穿化学防护服。不要直接接触泄漏物,用砂土吸收,铲入提桶,倒至空旷地方深埋。在污染区撤上石灰,用大量水冲洗,经稀释的污水放入废水系统。如果大量泄漏,小心扫起,装入备用袋中。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,提供充分的局部排风。防止粉尘释放到车间空气中。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴防尘面具(全面罩),穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂、酸类、碱类接触。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。防止阳光直射。包装密封。应与氧化剂、酸类、碱类分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 密闭操作,局部排风。 |
| 呼吸系统防护: | 生产操作或农业使用时,佩戴防尘口罩。空气中浓度较高时,应该佩戴防毒面具。 |
| 眼睛防护: | 空气中浓度较高时,戴化学安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 戴防护手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋浴更衣。单独存放被毒物污染的衣服,洗后再用。工作服不要带到非 |
| 第九部分:理化特性 |
| 外观与性状: | 原药为乳白色无味非挥发性结晶。 |
| pH: | |
| 熔点(℃): | 39.5~41.5 |
| 沸点(℃): | 100/0.00266kPa |
| 相对密度(水=1): | 1.133(25/15.6℃) |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | 0.29×(10-5)/24℃ |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 14 H 20 ClNO 2 |
| 分子量: | 269.80 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 难溶于水,溶于乙醚、丙酮、苯、氯仿、乙醇。 |
| 主要用途: | 用作农用除草剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强酸、强碱。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氮氧化物、氯化氢。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:930mg/kg(大鼠经口);13300mg/kg(兔经皮) LC50:1040mg/m3(大鼠吸入) |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 用安全掩埋法处置。在规定场所掩埋空容器。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 61900 |
| UN编号: | 2769 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | 塑料袋或二层牛皮纸袋外全开口或中开口钢桶;两层塑料袋或一层塑料袋外麻袋、塑料编织袋、乳胶布袋;塑料袋外复合塑料编织袋(聚丙烯三合一袋、聚乙烯三合一袋、聚丙烯二合一袋、聚乙烯二合一袋);塑料袋或二层牛皮纸袋外普通木箱;螺纹口玻璃瓶、塑料瓶、复合塑料瓶或铝瓶外普通木箱;塑料瓶、两层塑料袋或两层牛皮纸袋(内或外套以塑料袋)外瓦楞纸箱。 |
| 运输注意事项: | 铁路运输时包装所用的麻袋、塑料编织袋、复合塑料编织袋的强度应符合国家标准要求。运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与酸类、氧化剂、食品及食品添加剂混运。运输时运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。运输途中应防曝晒、雨淋,防高温。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定;常用危险化学品的分类及标志 (GB 13690-92)将该物质划为第6.1 类毒害品。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 5 |
| MSDS修改日期: | 年月日 |
制备方法与用途
甲草胺又称拉索、杂草锁、草不绿,是一种酰胺类内吸选择性除草剂。适用于大豆、花生、棉花、玉米、油菜、小麦及蔬菜等作物使用,防除多种一年生禾本科杂草和苋、藜等阔叶杂草,对菟丝子也有一定的防效。
制法甲草胺的合成方法如下:2,6-二乙基苯胺与甲醛水溶液反应生成物再与氯乙酰氯反应,生成加成物。该加成物在氨作用下与甲醇反应,进行甲酰化,最终制得甲草胺。
毒性大鼠急性经口LD50为1200mg/kg,家兔急性经皮LD50为5000mg/kg(13300mg/kg);大鼠急性吸入LC50>1.04mg/L。大鼠90d饲喂试验无作用剂量为每天17mg/kg,慢性无作用剂量2.5mg/kg。无致畸、致突变作用,小鼠在15mg/kg和240~260mg/kg剂量下出现支气管肺泡肿瘤和肝、肺肿瘤。鲤鱼LC503.72mg/L。
化学性质甲草胺为奶油色固体。
用途甲草胺是一种选择性旱地芽前除草剂,植物幼芽吸收药剂后,抑制蛋白酶的活力,阻碍蛋白质合成,致使杂草死亡。主要用于在出苗前土壤中萌发的杂草,对已出土杂草基本无效。防除大豆、棉花、甜菜、玉米、花生、油菜等旱地作物田间的一年生禾本科杂草,如稗草、牛筋草、秋稷、马唐、狗尾草、蟋蟀草、臂形草等,用量一般为20~30g有效成分/100m²。如在大豆播种后至出苗前使用,用48%乳油30~45mL/100m²,对水均匀喷雾土表。如玉米、蔬菜、花生播种出苗前或移栽前使用,用48%乳油30~38mL/100m²,对水喷雾土表。
用途甲草胺被杂草的幼苗根部吸收,干扰核酸和蛋白质合成,阻止细胞增大,从而抑制根的伸长,然后导致整个植株死亡。主要用于防除一年生禾本科杂草及部分阔叶杂草,适用于多种作物田间使用。
制备方法甲草胺可以通过以下两种制备方法获得:
分类农药
- 毒性分级:高毒
-
急性毒性:
- 口服-大鼠 LD50: 930 毫克/ 公斤
- 口服-小鼠 LD50: 462 毫克/ 公斤
可燃性危险特性:燃烧产生有毒氮氧化物和氯化物气体。 储运特性:
- 库房通风低温干燥
- 与食品原料分开储运
灭火剂:
- 干粉
- 泡沫
- 砂土
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氯-N-(氯甲基)-N-(2,6-二乙基苯基)乙酰胺 2-chloro-N-chloromethyl-N-(2,6-diethylphenyl)acetamide 40164-69-0 C13H17Cl2NO 274.19 2-氯-2,6-二乙基乙酰苯胺 2-chloro-2',6'-diethylacetanilide 6967-29-9 C12H16ClNO 225.718 —— N-(Methoxymethyl)-2,6-diethylaniline 39180-88-6 C12H19NO 193.289 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 丁草胺 N-butoxymethyl-2-chloro-N-(2,6-diethylphenyl)acetamide 23184-66-9 C17H26ClNO2 311.852 —— N-(2,6-Diethylphenyl)-N-(methoxymethyl)acetamide 74886-79-6 C14H21NO2 235.326 2-羟基-2,6-二乙基-N-甲氧基甲基乙酰苯胺 2-Hydroxy-N-(2,6-diethylphenyl)-N-(methoxymethyl)acetamide 56681-55-1 C14H21NO3 251.326 2-氯-N-(氯甲基)-N-(2,6-二乙基苯基)乙酰胺 2-chloro-N-chloromethyl-N-(2,6-diethylphenyl)acetamide 40164-69-0 C13H17Cl2NO 274.19 —— Alachlor-ESA 142363-53-9 C14H21NO5S 315.39 —— Alachlor mercapturate —— C19H28N2O5S 396.508 2-氯-2,6-二乙基乙酰苯胺 2-chloro-2',6'-diethylacetanilide 6967-29-9 C12H16ClNO 225.718 异丙甲草胺 2-chloro-6'-ethyl-N-(2-methoxy-1-methylethyl)acet-o-toluidide 51218-45-2 C15H22ClNO2 283.798 2,6-二乙基-乙酰苯胺 2,6-diethylacetanilide 16665-89-7 C12H17NO 191.273 2-羟基-2',6'-二乙基乙酰苯胺 2-hydroxy-2',6'-diethylacetanilide 52559-52-1 C12H17NO2 207.272
反应信息
-
作为反应物:参考文献:名称:使用分子电催化剂对甲草胺进行加氢脱氯摘要:用于电催化氯化除草剂修复的分子催化剂:具有柔性三唑单元的钴卟啉络合物显示出对甲草胺加氢脱氯的电催化活性。DOI:10.1002/cctc.202201512
-
作为产物:描述:参考文献:名称:一种氯乙酰胺类化合物的合成方法摘要:本发明公开了一种氯乙酰胺类化合物的合成方法,在反应釜中,将仲胺化合物溶于有机溶剂,升温至回流,向其中加入氯乙酰氯,回流反应0.5~20个小时,得到氯乙酰胺类化合物,本发明的氯乙酰胺类化合物的合成方法,不使用缚酸剂,减少了后处理过程中废水的排放,通过保持体系的回流状态使反应生产的氯化氢气体排出体系,并在体系之外被水吸收,得到纯度较高的盐酸,变废为宝,并且符合安全环保的生产标准,减少了三废排放;本发明的氯乙酰胺类化合物的合成方法,操作步骤少,反应速度快,产品收率高,纯度高,生产成本低,安全环保,适合工业化的大规模生产。公开号:CN110117233A
-
作为试剂:描述:参考文献:名称:HDAC6 SELECTIVE INHIBITORS, PREPARATION METHOD THEREFOR, AND APPLICATION THEREOF摘要:公开号:EP3569592B1
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] 3-[(HYDRAZONO)METHYL]-N-(TETRAZOL-5-YL)-BENZAMIDE AND 3-[(HYDRAZONO)METHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE DERIVATIVES AS HERBICIDES<br/>[FR] DÉRIVÉS DE 3-[(HYDRAZONO))MÉTHYL]-N-(TÉTRAZOL-5-YL)-BENZAMIDE ET DE 3-[(HYDRAZONO)MÉTHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE UTILISÉS EN TANT QU'HERBICIDES申请人:SYNGENTA CROP PROTECTION AG公开号:WO2021013969A1公开(公告)日:2021-01-28The present invention related to compounds of Formula (I): or an agronomically acceptable salt thereof, wherein Q, R2, R3, R4, R5 and R6 are as described herein. The invention further relates to compositions comprising said compounds, to methods of controlling weeds using said compositions, and to the use of compounds of Formula (I) as a herbicide.本发明涉及以下式(I)的化合物或其农业上可接受的盐,其中Q、R2、R3、R4、R5和R6如本文所述。该发明还涉及包含所述化合物的组合物,使用这些组合物控制杂草的方法,以及将式(I)的化合物用作除草剂的用途。
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
-
[EN] BLUE POLYMERIC HAIR DYES<br/>[FR] COLORANTS CAPILLAIRES POLYMÈRES BLEUS申请人:CIBA HOLDING INC公开号:WO2009090124A1公开(公告)日:2009-07-23Disclosed are cationic polymeric dye with a hue value of h = 210° to 330° comprising: a) a polymer backbone, b) a residue of an organic dye, and c) optionally colorless organic groups, wherein (b) and (c) are covalently bound to the polymer backbone (a), and wherein the cationic charges can independently be part of the dye or the colorless organic groups. The dyes are distinguished by their depth of shade and their good fastness properties to washing, such as, for example, fastness to light, shampooing and rubbing.
-
[EN] HERBICIDALLY ACTIVE HETEROARYL-S?BSTIT?TED CYCLIC DIONES OR DERIVATIVES THEREOF<br/>[FR] DIONES CYCLIQUES SUBSTITUÉES PAR HÉTÉROARYLE À ACTIVITÉ HERBICIDE OU DÉRIVÉS DE CELLES-CI申请人:SYNGENTA LTD公开号:WO2011012862A1公开(公告)日:2011-02-03The invention relates to a compound of formula (I), which is suitable for use as a herbicide wherein G is hydrogen or an agriculturally acceptable metal, sulfonium, ammonium or latentiating group; Q is a unsubstituted or substituted C3-C8 saturated or mono-unsaturated heterocyclyl containing at least one heteroatom selected from O, N and S, or Q is heteroaryl or substituted heteroaryl; m is 1, 2 or 3; and Het is an optionally substituted monocyclic or bicyclic heteroaromatic ring; and wherein the compound is optionally an agronomically acceptable salt thereof.
表征谱图
-
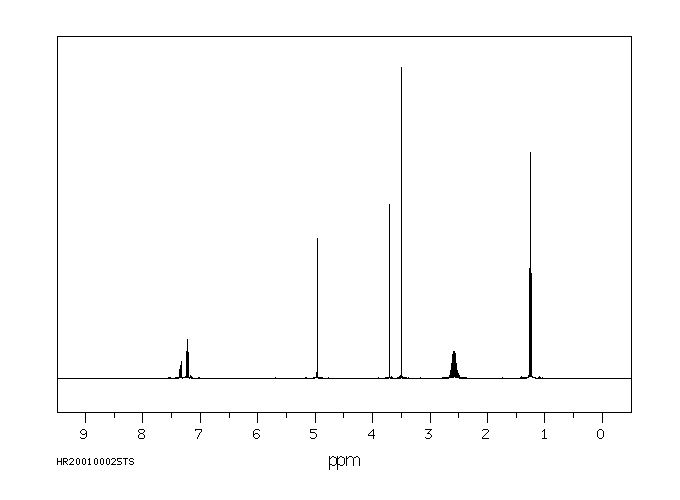
氢谱1HNMR
-
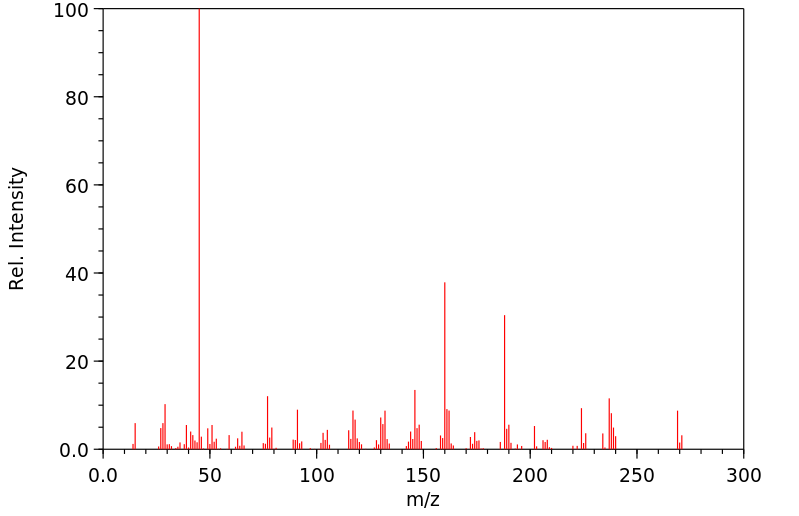
质谱MS
-
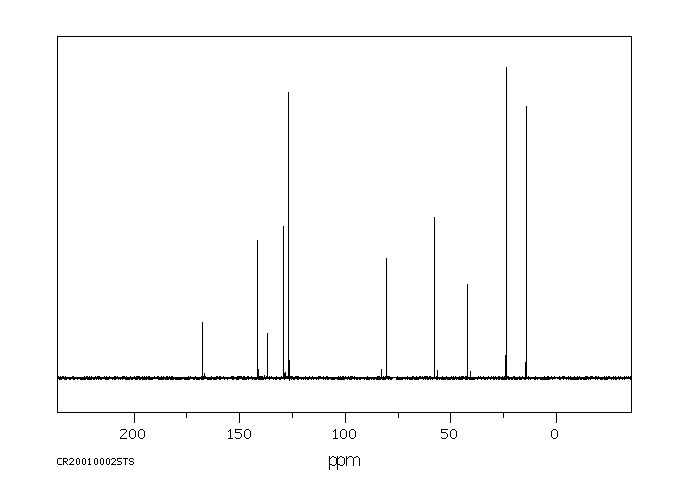
碳谱13CNMR
-
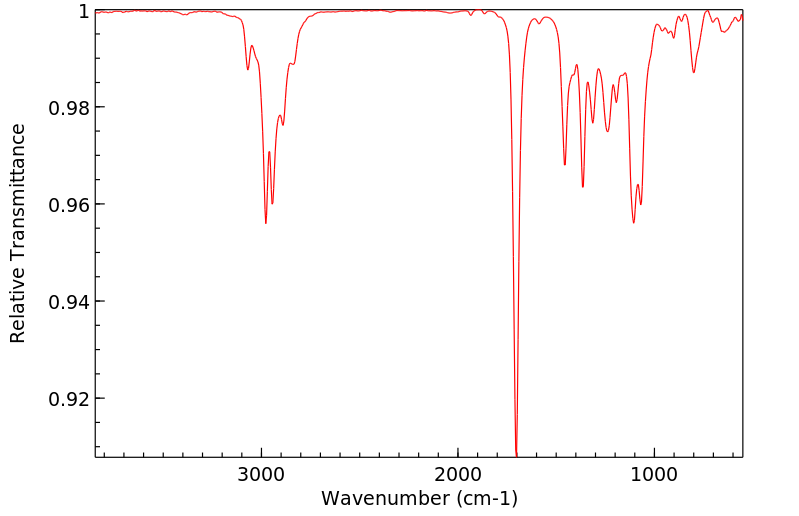
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息