3-氨基-N,N-二甲基苯甲酰胺 | 33322-60-0
中文名称
3-氨基-N,N-二甲基苯甲酰胺
中文别名
——
英文名称
3-amino-N,N-dimethylbenzamide
英文别名
N,N-Dimethyl-m-amino-benzamid
CAS
33322-60-0
化学式
C9H12N2O
mdl
MFCD01168905
分子量
164.207
InChiKey
LZPLRAXAVPPVSX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:87-88 °C
-
沸点:351.6±25.0 °C(Predicted)
-
密度:1.116±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:46.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2924299090
-
危险标志:GHS07
-
危险性描述:H317
-
危险性防范说明:P280
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 3-Amino-n,n-dimethylbenzamide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3-Amino-n,n-dimethylbenzamide
CAS number: 33322-60-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H12N2O
Molecular weight: 164.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 3-Amino-n,n-dimethylbenzamide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3-Amino-n,n-dimethylbenzamide
CAS number: 33322-60-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H12N2O
Molecular weight: 164.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基-3-硝基苯甲酰胺 N,N-dimethyl-3-nitrobenzamide 7291-02-3 C9H10N2O3 194.19 —— tert-butyl 3-[(dimethylamino)carbonyl]phenylcarbamate 927206-91-5 C14H20N2O3 264.324 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-[(3-Aminopropyl)amino]-N,N-dimethylbenzamide 73279-19-3 C12H19N3O 221.302 —— 3-acetamido-N,N-dimethylbenzamide 500160-02-1 C11H14N2O2 206.244 3-氨基-N,N-二甲基苄胺 3-((dimethylamino)methyl)aniline 27958-77-6 C9H14N2 150.224 3-[二(2-氯乙基)氨基]-N,N-二甲基苯甲酰胺 m-N-(Dichloraethylamino)-dimethyl-benzoesaeureamid 24813-06-7 C13H18Cl2N2O 289.205 —— phenyl 3-(dimethylcarbamoyl)phenylcarbamate 50699-54-2 C16H16N2O3 284.315
反应信息
-
作为反应物:描述:参考文献:名称:Hypoxia-selective antitumor agents. 3. Relationships between structure and cytotoxicity against cultured tumor cells for substituted N,N-bis(2-chloroethyl)anilines摘要:A series of aniline mustards with a wide range of electron-donating and -withdrawing substituents in the 3- and 4-positions has been synthesized and evaluated for cytotoxicity in cell culture to examine the potential of using nitro group deactivated nitrogen mustards for the design of novel hypoxia-selective anticancer drugs (Denny, W. A.; Wilson, W. R. J. Med. Chem. 1986, 29, 879). Hydrolytic half-lives in tissue culture media, determined by bioassay against a cell line (UV4) defective in the repair of DNA interstrand cross-links showed the expected dependence on the Hammett electronic parameter, sigma, varying from 0.13 h for the 4-amino analogue to greater than 100 h for analogues with strongly electron-withdrawing substituents. Cytotoxic potencies in aerobic UV4 cultures showed a similar dependence on sigma. This dependence predicted that the 4-nitroaniline mustard would be 7200-fold less potent than its potential six-electron reduction product, the 4-amino compound, in growth inhibition assays using a 1-h drug exposure. The measured differential was much lower (225-fold) because of the instability of the latter compound, but a differential of 17,500-fold was observed in the initial rate of killing by using a clonogenic assay. The potential for formation of reactive mustards by reduction to the amine or hydroxylamine was demonstrated by the 4-nitroso compound, which had an aerobic toxicity similar to that of the amine. Although these features confirmed the original rationale, the 3-nitro- and 4-nitroaniline mustards had only minimal hypoxic selectivity against UV cells. Toxicity to hypoxic cells appears to be limited by the low reduction potentials of these compounds and consequent lack of enzymatic nitroreduction. However, this study has demonstrated that nitro groups can be used to latentiate aromatic nitrogen mustards and indicates that examples with higher reduction potentials could provide useful hypoxia-selective therapeutic agents.DOI:10.1021/jm00163a019
-
作为产物:描述:间氨基苯甲酸 在 盐酸 、 N,N'-羰基二咪唑 、 sodium hydroxide 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 二氯甲烷 、 水 为溶剂, 反应 4.0h, 生成 3-氨基-N,N-二甲基苯甲酰胺参考文献:名称:Novel Compounds for the Treatment of Diseases Associated with Amyloid or Amyloid-Like Proteins摘要:本发明涉及一种新型化合物,可用于治疗与淀粉样蛋白相关的一组疾病和异常,如阿尔茨海默病,以及与淀粉样蛋白类似蛋白相关的疾病或病况。本发明的化合物还可用于治疗与视觉系统组织中的病理异常/变化相关的眼部疾病。本发明还涉及包含这些化合物的药物组合物,以及利用这些化合物制备用于治疗或预防与淀粉样和/或淀粉样类似蛋白相关的疾病或病况的药物的用途。还公开了一种治疗或预防与淀粉样和/或淀粉样类似蛋白相关的疾病或病况的方法。公开号:US20110280808A1
文献信息
-
Cationic Ir/Me-BIPAM-Catalyzed Asymmetric Intramolecular Direct Hydroarylation of α-Ketoamides作者:Tomohiko Shirai、Hajime Ito、Yasunori YamamotoDOI:10.1002/anie.201400147日期:2014.3.3Asymmetric intramolecular direct hydroarylation of α‐ketoamides gives various types of optically active 3‐substituted 3‐hydroxy‐2‐oxindoles in high yields with complete regioselectivity and high enantioselectivities (84–98 % ee). This is realized by the use of the cationic iridium complex [Ir(cod)2](BArF4) and the chiral O‐linked bidentate phosphoramidite (R,R)‐Me‐BIPAM.
-
2-Arylamino-6-ethynylpurines are cysteine-targeting irreversible inhibitors of Nek2 kinase作者:Christopher J. Matheson、Christopher R. Coxon、Richard Bayliss、Kathy Boxall、Benoit Carbain、Andrew M. Fry、Ian R. Hardcastle、Suzannah J. Harnor、Corine Mas-Droux、David R. Newell、Mark W. Richards、Mangaleswaran Sivaprakasam、David Turner、Roger J. Griffin、Bernard T. Golding、Céline CanoDOI:10.1039/d0md00074d日期:——Renewed interest in covalent inhibitors of enzymes implicated in disease states has afforded several agents targeted at protein kinases of relevance to cancers. We now report the design, synthesis and biological evaluation of 6-ethynylpurines that act as covalent inhibitors of Nek2 by capturing a cysteine residue (Cys22) close to the catalytic domain of this protein kinase. Examination of the crystal人们对与疾病状态有关的酶的共价抑制剂重新产生了兴趣,从而提供了几种针对与癌症相关的蛋白激酶的药物。我们现在报告了 6-乙炔基嘌呤的设计、合成和生物学评价,6-乙炔基嘌呤通过捕获靠近该蛋白激酶催化结构域的半胱氨酸残基 (Cys22) 来充当 Nek2 的共价抑制剂。对与 Nek2 复合的非共价抑制剂 3-((6-环己基甲氧基-7 H-嘌呤-2-基)氨基)苯甲酰胺的晶体结构的检查表明,用乙炔基取代烷氧基放置了炔烃的末端接近Cys22并且处于与迈克尔加成的立体电子学要求兼容的位置。制备了一系列 6-乙炔基嘌呤并建立了抑制 Nek2 的结构活性关系 (SAR)。 6-乙炔基-N-苯基-7 H-嘌呤-2-胺 [IC 50 0.15 μM (Nek2)] 和 4-((6-乙炔基-7 H-嘌呤-2-基)氨基)苯磺酰胺 (IC 50 0.14)选择 Nek2 的抑制模式来确定 Nek2 的抑制模式,该抑制模式具有时间依赖性,不能通过添加
-
NOVEL LOW-MOLECULAR-COMPOUND FOR IMPROVING PRODUCTION, MAINTENANCE AND PROLIFERATION OF PLURIPOTENT STEM CELLS, COMPOSITION COMPRISING THE SAME, AND CULTURE METHOD申请人:KOREA RESEARCH INSTITUTE OF BIOSCIENCE AND BIOTECHNOLOGY公开号:US20150159142A1公开(公告)日:2015-06-11According to the present invention, when the novel low-molecular-weight compound RSC-133 is added in a culture process for producing reprogrammed pluripotent stem cells from human differentiated cells, it can increase the efficiency of reprogramming and can significantly reduce the time required for the induction of reprogramming. Particularly, the novel compound RSC-133 can substitute for c-Myc acting as both a reprogramming factor and an oncogenic factor, and it can effectively increase the efficiency of reprogramming in both normal oxygen culture conditions and hypoxic culture conditions. In addition, RSC-133 can inhibit the induction of aging occurring in the reprogramming process, exhibits the effect of promoting cell proliferation, and induces epigenetic activation to improve culture conditions for induction of reprogramming. The present invention will contribute to optimizing a process of producing induced pluripotent stem cells from a small amount of patient-specific somatic cells obtained from various sources, and thus it will significantly improve a process of developing clinically applicable personalized stem cell therapy agents and new drugs and will facilitate the practical use of these agents and drugs. In addition, the novel low-molecular-weight compound RSC-133 can provide a cell culture medium effective for maintaining the undifferentiated state of human embryonic stem cells that are typical pluripotent stem cells. The medium composition containing RSC-133 can effectively induce the proliferation of human embryonic stem cells in an undifferentiated state and can be effectively used for the development of a system for culturing large amounts of embryonic stem cells.根据本发明,当在从人类分化细胞生产重编程的多能干细胞的文化过程中添加新型的低分子量化合物RSC-133时,它可以提高重编程的效率,并且可以显著减少诱导重编程所需的时间。特别是,新型化合物RSC-133可以替代作为重编程因子和致癌因子的c-Myc,并且可以在正常氧文化条件和无氧文化条件下有效地提高重编程的效率。此外,RSC-133可以抑制在重编程过程中发生的衰老诱导,表现出促进细胞增殖的效果,并诱导表观遗传激活以改善重编程诱导的培养条件。本发明将有助于优化从各种来源获得的患者特异性体细胞的小量生产诱导多能干细胞的过程,从而将显著改进开发临床应用的个人化干细胞治疗剂和新药的过程,并将促进这些剂和药物的实用化。另外,新型的低分子量化合物RSC-133可以提供一个有效的细胞培养介质,用于维持典型多能干细胞的人胚胎干细胞的不分化状态。含有RSC-133的培养基质可以有效诱导人胚胎干细胞在不分化状态的增殖,并且可以有效地用于大量胚胎干细胞培养系统的开发。
-
[EN] COMPOUNDS AND COMPOSITIONS FOR TREATING CONDITIONS ASSOCIATED WITH NLRP ACTIVITY<br/>[FR] COMPOSÉS ET COMPOSITIONS DESTINÉS AU TRAITEMENT D'ÉTATS PATHOLOGIQUES ASSOCIÉS À UNE ACTIVITÉ DE NLRP申请人:IFM TRE INC公开号:WO2019023147A1公开(公告)日:2019-01-31In one aspect, compounds of Formula AA, or a pharmaceutically acceptable salt thereof, are featured.The variables shown in Formula AA are as defined in the claims. The compounds of formula AA are NLRP3 activity modulators and, as such, can be used in the treatment of metabolic disorders (e.g. Type 2 diabetes, atherosclerosis, obesity or gout), a disease of the central nervous system (e.g. Alzheimer's disease, multiple sclerosis, Amyotrophic Lateral Sclerosis or Parkinson's disease), lung disease (e.g. asthma, COPD or pulmonary idiopathic fibrosis), liver disease (e.g. NASH syndrome, viral hepatitis or cirrhosis), pancreatic disease (e.g. acute pancreatitis or chronic pancreatitis), kidney disease (e.g. acute kidney injury or chronic kidney injury), intestinal disease (e.g. Crohn's disease or Ulcerative Colitis), skin disease (e.g. psoriasis), musculoskeletal disease (e.g. scleroderma), a vessel disorder (e.g. giant cell arteritis), a disorder of the bones (e.g. osteoarthritis, osteoporosis or osteopetrosis disorders), eye disease (e.g. glaucoma or macular degeneration), a disease caused by viral infection (e.g. HIV or AIDS), an autoimmune disease (e.g. Rheumatoid Arthritis, Systemic Lupus Erythematosus or Autoimmune Thyroiditis), cancer or aging.在一方面,特征在于公式AA的化合物,或其药用可接受的盐。公式AA中所示的变量如权利要求中所定义。公式AA的化合物是NLRP3活性的调节剂,因此,可用于治疗代谢紊乱(例如2型糖尿病、动脉硬化、肥胖或痛风)、中枢神经系统疾病(例如阿尔茨海默病、多发性硬化症、肌萎缩侧索硬化症或帕金森病)、肺病(例如哮喘、慢性阻塞性肺病或特发性肺纤维化)、肝病(例如非酒精性脂肪肝炎、病毒性肝炎或肝硬化)、胰腺病(例如急性胰腺炎或慢性胰腺炎)、肾病(例如急性肾损伤或慢性肾损伤)、肠病(例如克罗恩病或溃疡性结肠炎)、皮肤病(例如银屑病)、肌肉骨骼疾病(例如硬皮病)、血管障碍(例如巨细胞动脉炎)、骨骼疾病(例如骨关节炎、骨质疏松症或骨石化病)、眼病(例如青光眼或黄斑变性)、由病毒感染引起的疾病(例如HIV或艾滋病)、自身免疫病(例如类风湿性关节炎、系统性红斑狼疮或自身免疫性甲状腺炎)、癌症或衰老。
-
[EN] TRICYCLIC HETEROARYL-SUBSTITUTED QUINOLINE AND AZAQUINOLINE COMPOUNDS AS PAR4 INHIBITORS<br/>[FR] COMPOSÉS TRICYCLIQUES DE QUINOLÉINE ET D'AZAQUINOLINE À SUBSTITUTION HÉTÉROARYLE INHIBITEURS DE PAR4申请人:BRISTOL MYERS SQUIBB CO公开号:WO2018013776A1公开(公告)日:2018-01-18Disclosed are compounds of Formula (I) to (VIII): (I) (II) (III) (IV) (V) (VI) (VII) (VIII) or a stereoisomer, tautomer, pharmaceutically acceptable salt, solvate or prodrug thereof, wherein R3 is a tricyclic heteroaryl group substituted with R3a and zero to 2 R3b; and R1, R2, R3a, R3b, R4, and n are defined herein. Also disclosed are methods of using such compounds as PAR4 inhibitors, and pharmaceutical compositions comprising such compounds. These compounds are useful in inhibiting or preventing platelet aggregation, and are useful for the treatment of a thromboembolic disorder or the primary prophylaxis of a thromboembolic disorder.
表征谱图
-
氢谱1HNMR
-
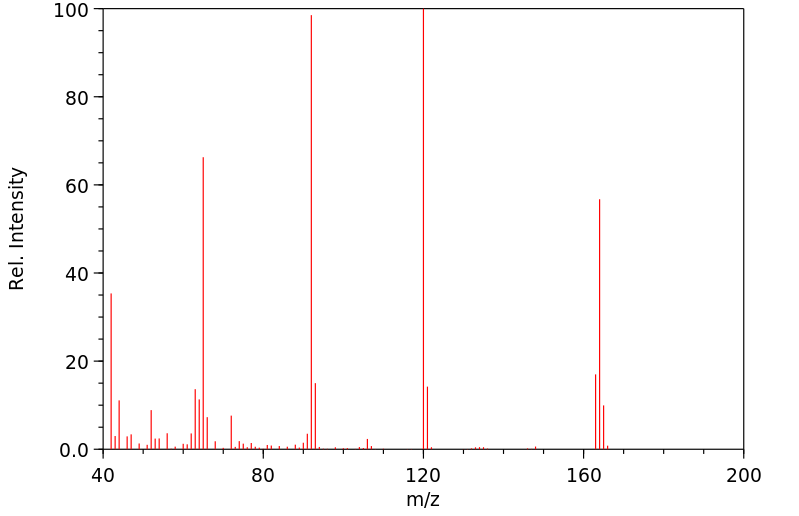
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫