3-醛基水杨酸 | 610-04-8
中文名称
3-醛基水杨酸
中文别名
——
英文名称
3-formylsalicylic acid
英文别名
3-formyl-2-hydroxybenzoic acid
CAS
610-04-8
化学式
C8H6O4
mdl
MFCD00143165
分子量
166.133
InChiKey
YOEUNKPREOJHBW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:177.5°C
-
沸点:214.32°C (rough estimate)
-
密度:1.2208 (rough estimate)
-
溶解度:溶于二甲基亚砜
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
海关编码:2918990090
-
储存条件:室温、密闭保存,并确保干燥。
SDS
| Name: | 3-Formylsalicylic acid 97% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 610-04-8 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 610-04-8 | 3-Formylsalicylic acid | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause digestive tract disturbances. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. If irritation develops, get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash hands before eating. Avoid breathing dust, vapor, mist, or gas.
Avoid contact with skin and eyes. Keep container tightly closed. Use with adequate ventilation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 610-04-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear a chemical apron. Wear appropriate clothing to prevent skin exposure.
Respirators:
A NIOSH/MSHA approved air purifying dust or mist respirator or European Standard EN 149. Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: off-white
Odor: acetic odor
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 180 - 183 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H6O4
Molecular Weight: 166.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, moisture.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 610-04-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Formylsalicylic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 610-04-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 610-04-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 610-04-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-羟基-3-(羟甲基)苯甲酸 2-hydroxy-3-hydroxymethyl-benzoic acid 93610-16-3 C8H8O4 168.149 水杨酸 salicylic acid 69-72-7 C7H6O3 138.123 2-羟基-3-丙烯基-苯甲酸 3-Propenyl-salicylsaeure 92810-83-8 C10H10O3 178.188 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-羟基间苯二甲酸 2-hydroxyisophthalic acid 606-19-9 C8H6O5 182.133 2-羟基-3-(羟甲基)苯甲酸 2-hydroxy-3-hydroxymethyl-benzoic acid 93610-16-3 C8H8O4 168.149 3-甲酰基-2-羟基苯甲酸甲酯 methyl 3-formyl-2-hydroxybenzoate 3775-05-1 C9H8O4 180.16 —— 3-formyl-2,5-dihydroxy-benzoic acid —— C8H6O5 182.133 —— 5-chloro-3-formyl-2-hydroxybenzoic acid 111870-27-0 C8H5ClO4 200.578 —— 2,6-bicarboxy-4-chloro-phenol 773869-56-0 C8H5ClO5 216.578 —— 3-bromo-5-formyl-6-hydroxybenzoic acid 134948-41-7 C8H5BrO4 245.029 3-甲醛基-2-甲氧基苯甲酸甲酯 methyl 3-formyl-2-methoxybenzoate 186312-96-9 C10H10O4 194.187 —— 3-hydroxyiminomethylsalicylic acid 7383-10-0 C8H7NO4 181.148 —— 3-(2-phenylethene)-salicylate 92496-80-5 C15H12O3 240.258 2-羟基间苯二甲醛 2-hydroxy-isophthalaldehyde 3328-69-6 C8H6O3 150.134 —— (3-carboxy-2-hydroxy-phenyl)-acetic acid —— C9H8O5 196.16 —— 3-formylsalicylic acid semicarbazone —— C9H9N3O4 223.188 苯甲酸,二羟基- 2,3-Dihydroxybenzoic acid 303-38-8 C7H6O4 154.122 —— 5-Nitro-3-carboxy-2-hydroxy-benzaldehyd 22948-26-1 C8H5NO6 211.131 —— 3-[(2-benzoyl-2-methylhydrazino)methyl]-2-hydroxybenzoic acid 330580-62-6 C16H16N2O4 300.314 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis, biological evaluation and 3D-QSAR studies of 3-keto salicylic acid chalcones and related amides as novel HIV-1 integrase inhibitors摘要:HIV-1 integrase is one of the three most important enzymes required for viral replication and is therefore an attractive target for anti retroviral therapy. We herein report the design and synthesis of 3-keto salicylic acid chalcone derivatives as novel HIV-1 integrase inhibitors. The most active compound, 5-bromo-2-hydroxy-3-[3-(2,3,6-trichlorophenyl)acryloyl] benzoic acid (25) was selectively active against integrase strand transfer, with an IC50 of 3.7 mu M. While most of the compounds exhibited strand transfer selectivity, a few were nonselective, such as 5-bromo-3-[3-(4-bromophenyl)acryloyl]-2-hydroxybenzoic acid (15), which was active against both 3'-processing and strand transfer with IC50 values of 11 +/- 4 and 5 +/- 2 mu M, respectively. The compounds also inhibited HIV replication with potencies comparable with their integrase inhibitory potencies. Thus, 5-bromo-2-hydroxy-3-[3-(2,3,6-trichlorophenyl) acryloyl] benzoic acid (25) and 5-bromo-3-[3-(4-bromophenyl) acryloyl]-2-hydroxybenzoic acid (15) inhibited HIV-1 replication with EC50 values of 7.3 and 8.7 mu M, respectively. A PHASE pharmacophore hypothesis was developed and validated by 3D-QSAR, which gave a predictive r(2) of 0.57 for an external test set of ten compounds. Phamacophore derived molecular alignments were used for CoMFA and CoMSIA 3D-QSAR modeling. CoMSIA afforded the best model with q(2) and r(2) values of 0.54 and 0.94, respectively. This model predicted all the ten compounds of the test set within 0.56 log units of the actual pIC(50) values; and can be used to guide the rational design of more potent novel 3-keto salicylic acid integrase inhibitors (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2011.01.047
-
作为产物:参考文献:名称:A novel sensitive and selective chemosensor for fluorescent detection of Zn2+ in cosmetics creams based on a covalent post functionalized Al-MOF摘要:基于席夫碱反应制备了一种材料,以实现NH2-MIL-53(Al)和3-甲醛基水杨酸的共价连接,用于基于CN异构化和ESIPT的抑制和破坏来荧光检测Zn2+离子。DOI:10.1039/d1nj00871d
文献信息
-
Small-molecules that covalently react with a human prolyl hydroxylase – towards activity modulation and substrate capture作者:Jacob T. Bush、Robert K. Leśniak、Tzu-Lan Yeh、Roman Belle、Holger Kramer、Anthony Tumber、Rasheduzzaman Chowdhury、Emily Flashman、Jasmin Mecinović、Christopher J. SchofieldDOI:10.1039/c8cc07706a日期:——
We describe covalently binding modulators of the activity of human prolyl hydroxylase domain 2 (PHD2) and studies towards a strategy for photocapture of PHD2 substrates.
我们描述了共价结合的人类脯氨酸羟化酶结构域2(PHD2)活性调节剂,并进行了研究,以制定一种用于光捕获PHD2底物的策略。 -
Synthesis and fluorescence properties of benzoxazole-1,4-dihydropyridine dyads achieved by a multicomponent reaction作者:Ricardo Ferreira Affeldt、Antônio César de Amorim Borges、Dennis Russowsky、Fabiano Severo RodembuschDOI:10.1039/c4nj00777h日期:——
Photoactive ESIPT dyads with a high Stokes' shift were obtained by a multicomponent one-pot Hantzsch synthesis.
具有高Stokes位移的光活性ESIPT双子通过多组分一锅Hantzsch合成获得。 -
Synthesis and Characterisation of Polymer‐Anchored Oxidovanadium(IV) Complexes and Their Use for the Oxidation of Styrene and Cumene作者:Mannar R. Maurya、Umesh Kumar、P. ManikandanDOI:10.1002/ejic.200601130日期:2007.6PS-H2fsal-ea (IV), PS-H2fsal-pa (V) and PS-H2fsal-amp (VI) gave the oxidovanadium(IV) complexes, PS-[VO(fsal-ea)·DMF] (4), PS-[VO(fsal-pa)·DMF] (5) and PS-[VO(fsal-amp)·DMF] (6), respectively. The corresponding neat complexes [VO(fsal-ea)]2 (1), [VO(fsal-pa)]2 (2) and [VO(fsal-amp)]2 (3) have also been similarly prepared. These complexes all exhibit a medium intensity band between 964 and 993 cm–1 in theirSchiff 碱 H2fsal-ea (I)、H2fsal-pa (II) 和 H2fsal-amp (III),分别衍生自 3-甲酰基水杨酸和 2-氨基乙醇、3-氨基丙醇和 2-氨基-2-甲基丙醇,分别具有通过共价键连接到用 5% 二乙烯基苯交联的氯甲基化聚苯乙烯。在二甲基甲酰胺 (DMF) 中用 [VO(acac)2] 处理后,这些聚合物锚定的配体 PS-H2fsal-ea (IV)、PS-H2fsal-pa (V) 和 PS-H2fsal-amp (VI) 得到氧化钒(IV) 复合物,PS-[VO(fsal-ea)·DMF] (4), PS-[VO(fsal-pa)·DMF] (5) 和 PS-[VO(fsal-amp)·DMF] ( 6) 分别。相应的纯配合物 [VO(fsal-ea)]2 (1)、[VO(fsal-pa)]2 (2) 和 [VO(fsal-amp)]2 (3) 也已类似地制备。由于
-
Biarylmethyl indolines, indoles and tetrahydroquinolines, useful as serine protease inhibitors申请人:——公开号:US20040220206A1公开(公告)日:2004-11-04The present invention provides compounds of Formula (I): 1 or a stereoisomer or pharmaceutically acceptable salt or hydrate form thereof, wherein the variables A, B, L 1 , L 2 , X 1 , X 2 , X 3 , X 4 and W are as defined herein. The compounds of Formula (I) are useful as selective inhibitors of serine protease enzymes of the coagulation cascade and/or contact activation system; for example thrombin, factor Xa, factor XIa, factor IXa, factor VIIa and/or plasma kallikrein. In particular, it relates to compounds that are selective factor XIa inhibitors. This invention also relates to pharmaceutical compositions comprising these compounds and methods of treating thromboembolic and/or inflammatory disorders using the same.
-
The synthesis and evaluation of benzofuranones as β-Lactamase substrates作者:S AdediranDOI:10.1016/s0968-0896(00)00345-x日期:2001.5These compounds were prepared by lactonization of the corresponding, appropriately substituted phenylglycines. The latter compounds were prepared by either the Strecker or the Bücherer-Berg method. The benzofuran-2-ones were less stable in aqueous solution than the analogous acyclic phenaceturate esters but comparably stable to analogous benzopyran-2-ones. They differed from the latter compounds however已经合成了6-和7-羧基-3-苯基乙酰胺基-3H-1-苯并呋喃-2-酮作为潜在的β-内酰胺酶底物和/或抑制剂。这些化合物通过相应地适当取代的苯基甘氨酸的内酯化制备。后一种化合物是通过Strecker或Bücherer-Berg方法制备的。苯并呋喃-2-酮在水溶液中的稳定性不及类似的无环苯乙酸酯,但与类似的苯并吡喃-2-酮相比则稳定。但是,它们与后一种化合物的区别在于,呋喃-2-酮的C-3氢与内酯羰基相邻,具有明显的酸性。7-羧基-3-苯基乙酰胺基-3H-1-苯并呋喃-2-酮在pH 7.5下以烯醇化物的形式大量存在。呋喃-2-酮是β-内酰胺酶的底物,其反应性与类似的无环苯乙酸盐非常相似。但是,它们不是DD肽酶抑制剂,因此不太可能具有抗生素活性。讨论了这些观察的结构基础。
表征谱图
-
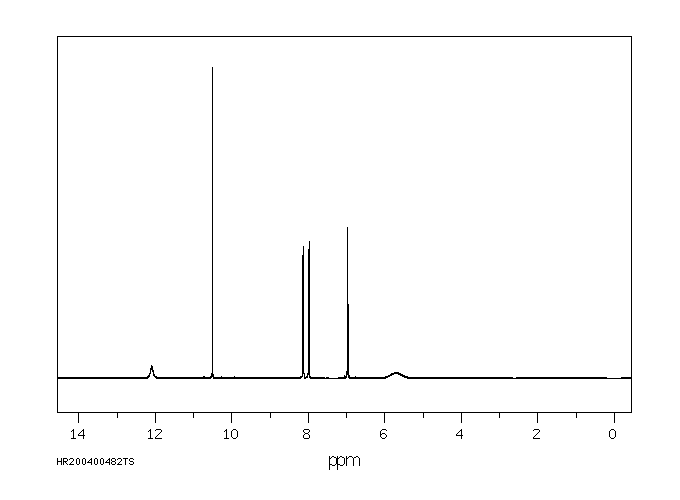
氢谱1HNMR
-
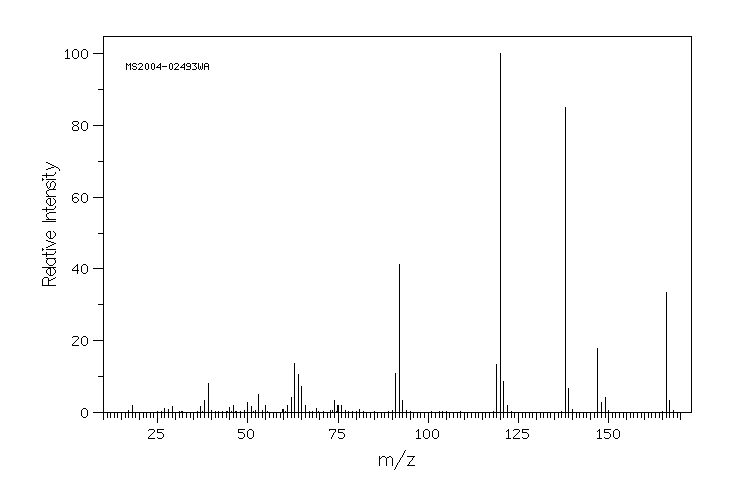
质谱MS
-
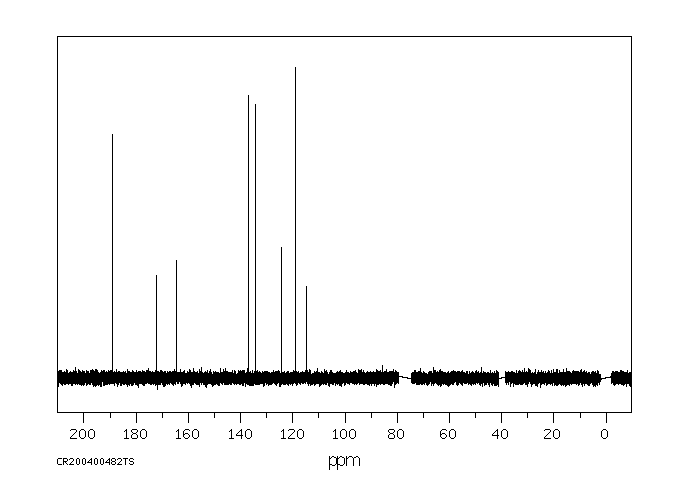
碳谱13CNMR
-
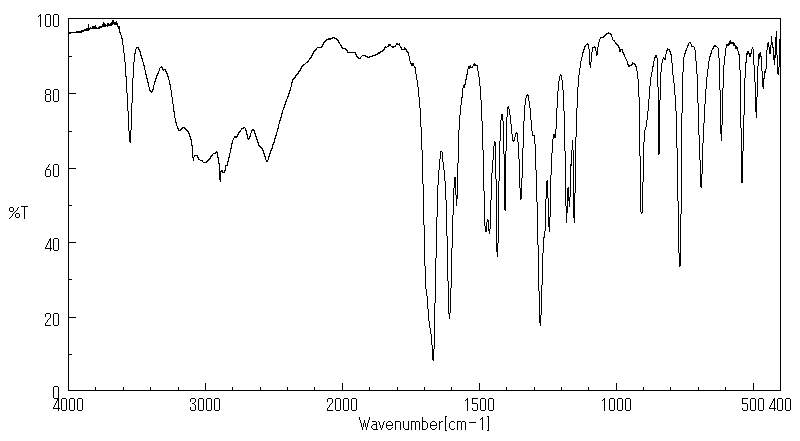
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫