4,4'-联吡啶二硫醚 | 2645-22-9
中文名称
4,4'-联吡啶二硫醚
中文别名
4,4'-二吡啶二硫醚;4,4'-二吡啶二硫;4,4'-二硫代联吡啶;4,4'-二硫化二吡啶;4,4'-二硫双吡啶;4,4'-二吡啶基二硫;4,4’-二吡啶二硫;4,4'-二吡啶基二硫;4,4"-二吡啶二硫醚;4,4-二吡啶二硫
英文名称
bis(4-pyridyl) disulfide
英文别名
dipyridin-4-yl disulfide;4,4'-dithiodipyridine;4,4'-dipyridyldisulfide;4,4'-bipyridyl disulfide;4,4′-dithiodipyridine;4,4’-dithiodipyridine;4,4'-Dipyridyl disulfide;4-(pyridin-4-yldisulfanyl)pyridine
CAS
2645-22-9
化学式
C10H8N2S2
mdl
MFCD00006423
分子量
220.319
InChiKey
UHBAPGWWRFVTFS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:76-78 °C(lit.)
-
沸点:356.1±17.0 °C(Predicted)
-
密度:1.3078 (rough estimate)
-
溶解度:95%乙醇:可溶,5%,澄清,无色至浅黄色
-
稳定性/保质期:
如果按照规格进行使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:76.4
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933399090
-
危险品运输编号:NONH for all modes of transport
-
储存条件:密封于0-6℃阴凉干燥环境中。
SDS
4,4'-二吡啶二硫醚 修改号码:2
模块 1. 化学品
产品名称: 4,4'-Dipyridyl Disulfide
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二吡啶二硫醚
百分比: >97.0%(GC)(T)
CAS编码: 2645-22-9
俗名: 4,4'-Dithiodipyridine
分子式: C10H8N2S2
4,4'-二吡啶二硫醚 修改号码:2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷藏储存。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅红黄色
4,4'-二吡啶二硫醚 修改号码:2
模块 9. 理化特性
气味: 无资料
pH: 无数据资料
熔点:
75°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
避免接触的条件: 热敏, 气敏
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
4,4'-二吡啶二硫醚 修改号码:2
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4,4'-Dipyridyl Disulfide
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二吡啶二硫醚
百分比: >97.0%(GC)(T)
CAS编码: 2645-22-9
俗名: 4,4'-Dithiodipyridine
分子式: C10H8N2S2
4,4'-二吡啶二硫醚 修改号码:2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷藏储存。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅红黄色
4,4'-二吡啶二硫醚 修改号码:2
模块 9. 理化特性
气味: 无资料
pH: 无数据资料
熔点:
75°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
避免接触的条件: 热敏, 气敏
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
4,4'-二吡啶二硫醚 修改号码:2
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Boduszek, B., Polish Journal of Chemistry, 1992, vol. 66, # 5, p. 787 - 790摘要:DOI:
-
作为产物:描述:4-氯吡啶 在 sodium hydroxide 、 乙醇 、 水 、 碘 、 potassium hydrosulfide 、 potassium iodide 作用下, 生成 4,4'-联吡啶二硫醚参考文献:名称:Koenigs; Kinne, Chemische Berichte, 1921, vol. 54, p. 1359摘要:DOI:
-
作为试剂:描述:色胺 、 succinyl-CoA 在 4,4'-联吡啶二硫醚 、 wild-type arylalkylamine N-acyltransferase from Tribolium castaneum 作用下, 以 aq. buffer 为溶剂, 生成 Bernsteinsaeure-<2-(indolyl-(3))-ethylamid>参考文献:名称:来自 Tribolium castaneum 的芳烷基胺 N-酰基转移酶的表征:对潜在的下一代杀虫剂目标的调查。摘要:杀虫剂抗性问题日益严重,这意味着确定新的杀虫剂目标变得前所未有的重要。芳烷基胺 N-酰基转移酶 (AANATs) 已被建议作为潜在的新目标。这些混杂的酶参与生物胺的 N-酰化以形成 N-酰胺。在昆虫中,这个过程是黑色素、角质层硬化、生物胺去除和脂肪酸酰胺生物合成的关键步骤。表征的每个 AANAT 同种型的独特性质表明每个生物体都容纳了该生物体相对专有的离散 AANAT 组装。这意味着在杀虫剂设计中具有很高的选择性,同时也保持了多药性。此处介绍了对 AANAT 的全面动力学和结构分析,该分析在世界上所有植物商品中最常见的次生害虫之一 Tribolium castaneum 中发现。这种名为 TcAANAT0 的酶催化短链 N-酰基芳基烷基胺的形成,其中短链酰基辅酶 A (C2-C10)、苯甲酰辅酶 A 和琥珀酰辅酶 A 在酰基供体的作用下起作用。从大肠杆菌中表达和纯化重组 TcAANAT0,DOI:10.1021/acschembio.9b00973
文献信息
-
Synthesis of Sulfonimidamides from Sulfenamides via an Alkoxy‐amino‐λ <sup>6</sup> ‐sulfanenitrile Intermediate作者:Edward L. Briggs、Arianna Tota、Marco Colella、Leonardo Degennaro、Renzo Luisi、James A. BullDOI:10.1002/anie.201906001日期:2019.10few operationally simple methods for their preparation. Here, the synthesis of NH-sulfonimidamides is achieved directly from sulfenamides, themselves readily formed in one step from amines and disulfides. A highly chemoselective and one-pot NH and O transfer is developed, mediated by PhIO in iPrOH, using ammonium carbamate as the NH source, and in the presence of 1 equivalent of acetic acid. A wide磺酰胺类药物在医学和农业化学领域是一个令人着迷的新主题,并为磺酰胺类提供了有吸引力的生物等排体。但是,几乎没有操作上简单的方法来准备它们。在此,NH-亚磺酰胺的合成直接由亚磺酰胺完成,它们很容易在一个步骤中由胺和二硫化物形成。使用氨基甲酸铵作为NH源,在1当量的乙酸存在下,由iPrOH中的PhIO介导了高度化学选择性和一锅式NH和O转移。在发达的反应条件下可耐受多种官能团,这也使抗抑郁药desipramine和fluoxetine官能化,并制备了丙磺舒的氮杂类似物。该反应显示出通过不同且同时发生的机理途径进行,包括形成新型的S≡N磺腈作为中间体。用不同的醇制备了几种烷氧基-氨基-λ6-亚磺腈,并且显示出它们是一系列亲核试剂的烷基化剂。
-
Synthesis and characterization of oxo-vanadium complex anchored onto SBA-15 as a green, novel and reusable nanocatalyst for the oxidation of sulfides and oxidative coupling of thiols作者:Taiebeh Tamoradi、Mohammad Ghadermazi、Arash Ghorbani-Choghamarani、Somayeh MolaeiDOI:10.1007/s11164-018-3367-3日期:2018.7Abstract The present work describes the synthesis of a new oxo-vanadium complex immobilized on SBA-15 nanostructure as an efficient catalyst for oxidation of sulfides and oxidative coupling of thiols. Characterization of the resultant AMPD@SBA-15 nanostructure was performed by various physico-chemical techniques such as Fourier transform infrared spectroscopy, transmission and scanning electron microscopies摘要 本工作描述了固定在SBA-15纳米结构上的新型氧-钒配合物的合成,该配合物是硫化物氧化和硫醇氧化偶联的有效催化剂。所得的AMPD @ SBA-15纳米结构的表征是通过各种物理化学技术进行的,例如傅立叶变换红外光谱,透射和扫描电子显微镜,能量色散X射线光谱,电感耦合等离子体发射光谱,X射线衍射,热重分析和N 2吸附和解吸。所开发方法的结果带来了一些好处,例如使用可商购的,生态上无害的,操作简便的,廉价的和化学惰性的试剂。它显示出良好的反应时间,实用性和高效率,并且易于通过简单过滤从反应混合物中回收,并可以连续几个循环重复使用,而其催化活性没有明显变化。更重要的是,高效率,简单且廉价的方法,可商购的材料,易于分离以及环保的方法是当前采用的多相催化系统的几个优点。 图形概要
-
Hypervalent Iodine(III)-Promoted Metal-Free S-H Activation: An Approach for the Construction of S-S, S-N, and S-C Bonds作者:Eakkaphon Rattanangkool、Watanya Krailat、Tirayut Vilaivan、Preecha Phuwapraisirisan、Mongkol Sukwattanasinitt、Sumrit WacharasindhuDOI:10.1002/ejoc.201402180日期:2014.8thiols with (diacetoxyiodo)benzene (DIB) has been explored in the preparation of symmetrical disulfides and sulfenamides. Disulfides can be produced in excellent yields (75–95 %) upon treatment of thiols with DIB. The reaction was complete in less than five minutes at room temperature. Aliphatic, aromatic, and heteroaromatic thiols are compatible with this transformation. Moreover, heteroaromatic disulfides
-
A Mild, High-Yield Conversion of Thiols into Disulfides Using Disulfide Dication Salt: A New Redox System作者:Hisashi Fujihara、Ryouichi Akaishi、Naomichi FurukawaDOI:10.1246/bcsj.62.616日期:1989.2A new redox reaction of thiols with disulfide dication salt, 1,5-dithioniabicyclo[3.3.0]octane bis(trifluoromethanesulfonate), under mild conditions gave the corresponding disulfides in good yields together with 1,5-dithiacyclooctane.
-
Amino acid and water-driven tunable green protocol to access S–S/C–S bonds via aerobic oxidative coupling and hydrothiolation作者:Amit Shard、Rajesh Kumar、Saima Saima、Nidhi Sharma、Arun K. SinhaDOI:10.1039/c4ra02909g日期:——
Arginine in conjunction with water has been employed as an effective and recyclable organocatalyst for oxidative coupling of thiophenols and hydrothiolation of alkynes.
表征谱图
-
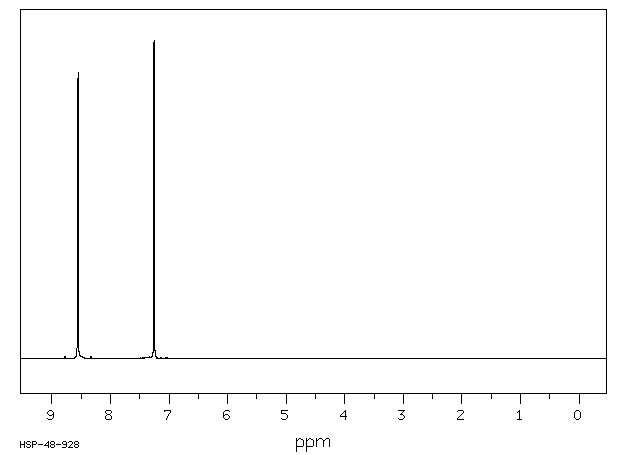
氢谱1HNMR
-
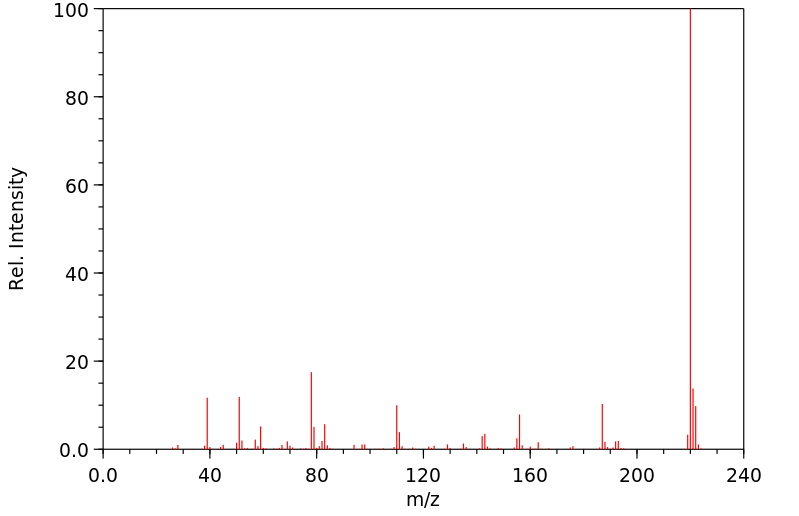
质谱MS
-
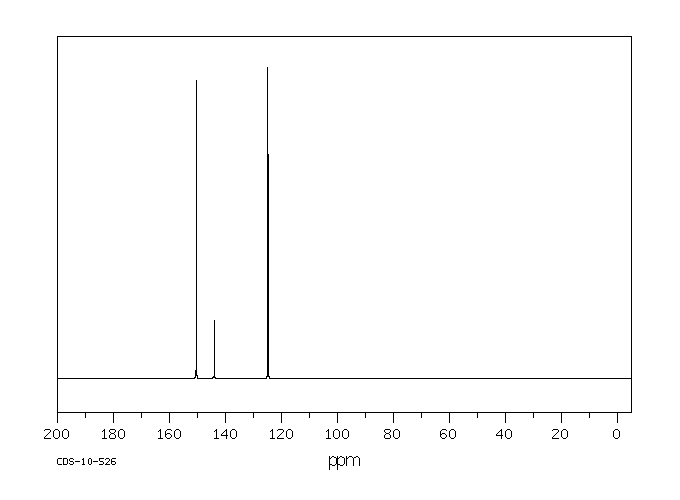
碳谱13CNMR
-
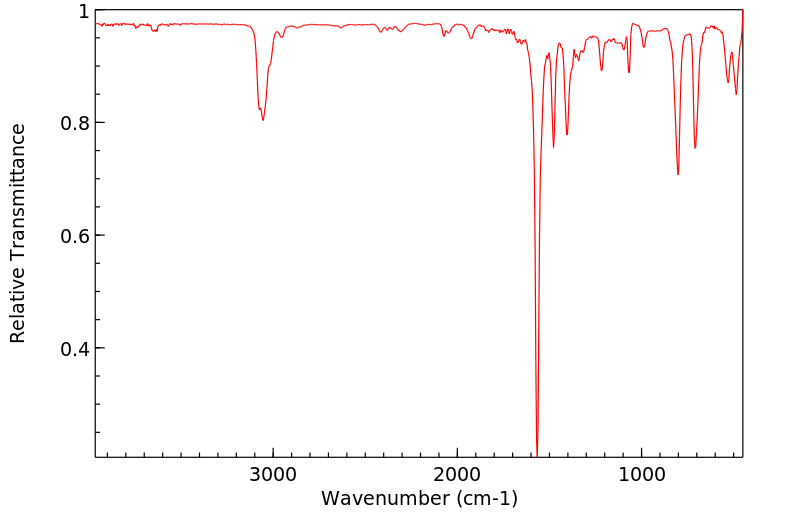
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-