红荧烯 | 517-51-1
中文名称
红荧烯
中文别名
红萤烯;5,6,11,12-四苯基并四苯;5,6,11,12-四苯基萘并萘;LT-E707
英文名称
5,6,11,12-tetraphenylnaphthacene
英文别名
Rubren;Rubrene;5,6,11,12-tetraphenyltetracene
CAS
517-51-1
化学式
C42H28
mdl
——
分子量
532.684
InChiKey
YYMBJDOZVAITBP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:330-335 °C (lit.)
-
沸点:>315°C
-
密度:1.1750 (estimate)
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):12.2
-
重原子数:42
-
可旋转键数:4
-
环数:8.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
安全说明:S22,S24/25
-
WGK Germany:3
-
海关编码:2902909090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:储存于阴凉、干燥、通风良好的库房。远离火种和热源,防止阳光直射,包装需密封。应与酸类及食用化学品分开存放,切忌混储。储区应备有合适的材料以收容泄漏物。
SDS
模块 1. 化学品
1.1 产品标识符
: 红荧烯
产品名称
1.2 鉴别的其他方法
5,6,11,12-Tetraphenylnaphthacene
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 5,6,11,12-Tetraphenylnaphthacene
别名
: C42H28
分子式
: 532.67 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 330 - 335 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:Acid-catalyzed transformation of rubrene to indenonaphthacene and its paired interacting orbital (PIO) analysis摘要:Treatment of rubrene (5,6,11,12-tetraphenylnaphthacene) with trifluoroacetic acid in dichloromethane gives 4b,9,10-triphenyl-4b,9-dihydroindeno[1,2,3-fg]naphthacene (1) as the sole isolable product. Paired interacting orbital (PIO) analysis indicates that the reaction is initiated by preferential attack of H+ at C(11) position of rubrene. (C) 2003 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(02)02831-9
-
作为产物:描述:5,6,11,12-四苯基-5,12-二氢-5,12-环氧四省 在 aluminum tri-bromide 、 cesium iodide 作用下, 以 氯仿 为溶剂, 以88%的产率得到红荧烯参考文献:名称:Regioselective synthesis of substituted rubrenes摘要:DOI:10.1021/jo00300a043
-
作为试剂:描述:参考文献:名称:An Optimised Procedure for PTFE Phase Vanishing Reactions: An Improved reaction Design and the use of Reagents Adsorbed on Silica摘要:虽然相消 (PV) - 聚四氟乙烯反应设计在多种基质和反应条件下都能很好地进行,但偶尔也会出现问题。本文讨论了这些问题及其重要性,包括它们对反应结果的影响以及解决这些问题的方法。此外,还介绍了一种改进设计的细节,即以前报道过的 PV-PTFE 和无溶剂 PV-PTFE 混合设计,以及二氧化硅支撑试剂的使用。DOI:10.3184/174751915x14416158938073
文献信息
-
The Rubrenic Synthesis: The Delicate Equilibrium between Tetracene and Cyclobutene作者:Daniele Braga、Abdelhafid Jaafari、Luciano Miozzo、Massimo Moret、Silvia Rizzato、Antonio Papagni、Abderrahim YassarDOI:10.1002/ejoc.201100033日期:2011.8we describe the synthesis of new substituted tetraaryltetracenes, obtained by the dimerization of triarylchloroallenes, prepared from propargyl alcohols. The propargyl alcohols were prepared by two different synthetic strategies and then the alcohols were treated to obtain the corresponding acenes. In addition to the expected tetracene derivatives, we observed the formation of bis(alkylidene)cyclobutenes
-
Preparative Oxidation of Organic Compounds in Microemulsions with Singlet Oxygen Generated Chemically by the Sodium Molybdate/Hydrogen Peroxide System<sup>1</sup>作者:Jean-Marie Aubry、Sabine BouttemyDOI:10.1021/ja9644079日期:1997.6.1designed to oxidize hydrophobic organic substrates with singlet oxygen (1O2, 1Δg) generated from the disproportionation of hydrogen peroxide catalyzed by molybdate ions. The microemulsion was prepared by mixing methylene chloride, sodium dodecylsulfate, n-butanol, and aqueous molybdate. Flash photolysis studies have shown that in such media singlet oxygen exhibits a similar kinetic behavior that under homogeneous
-
Calcium Peroxide Diperoxohydrate as a Storable Chemical Generator of Singlet Oxygen for Organic Synthesis作者:Christel Pierlot、Véronique Nardello、Jordane Schrive、Caroline Mabille、Jacques Barbillat、Bernard Sombret、Jean-Marie AubryDOI:10.1021/jo010766x日期:2002.4.1specific trapping have shown that CaO(2).2H(2)O(2) can be stored for several days at -80 degrees C and that the yield of (1)O(2) is equal to 25%. Oxidation of typical organic substrates in methanol or THF through [4 + 2] or [2 + 2] cycloaddition and ene reaction have been carried out on a preparative scale with total conversion and selectivity.
-
Rubrenes: Planar and Twisted作者:Abhimanyu S. Paraskar、A. Ravikumar Reddy、Asit Patra、Yair H. Wijsboom、Ori Gidron、Linda J. W. Shimon、Gregory Leitus、Michael BendikovDOI:10.1002/chem.200800802日期:——crystal, rubrene shows very low mobility in vacuum-sublimed or solution-processed organic thin-film transistors. We synthesized several rubrene analogues with electron-withdrawing and electron-donating substituents and found that most of the substituted rubrenes are not planar in the solid state. Moreover, we conclude (based on experimental and calculated data) that even parent rubrene is not planar in solution
-
Direct Near Infrared Light–Activatable Phthalocyanine Catalysts作者:Yoshino Katsurayama、Yasuhiro Ikabata、Hajime Maeda、Masahito Segi、Hiromi Nakai、Taniyuki FuruyamaDOI:10.1002/chem.202103223日期:2022.1.10Going in for infra red: The development of phthalocyanine catalysts directly activated by NIR light to transform small organic molecules is described. The choice of solvent is important to promote cross-dehydrogenative coupling under irradiation with 810 nm NIR light. A combined experimental and computational mechanistic analysis revealed that this NIR reaction involves a singlet-oxygen-mediated mechanism进入红外线:描述了开发由 NIR 光直接激活的酞菁催化剂以转化有机小分子。溶剂的选择对于在 810 nm 近红外光照射下促进交叉脱氢偶联很重要。结合实验和计算机制分析表明,这种 NIR 反应涉及单线态氧介导的能量转移机制。
表征谱图
-
氢谱1HNMR
-
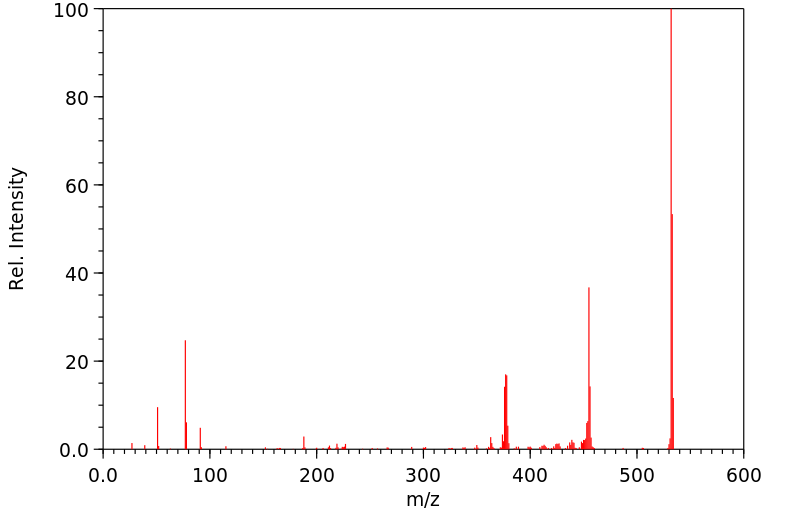
质谱MS
-
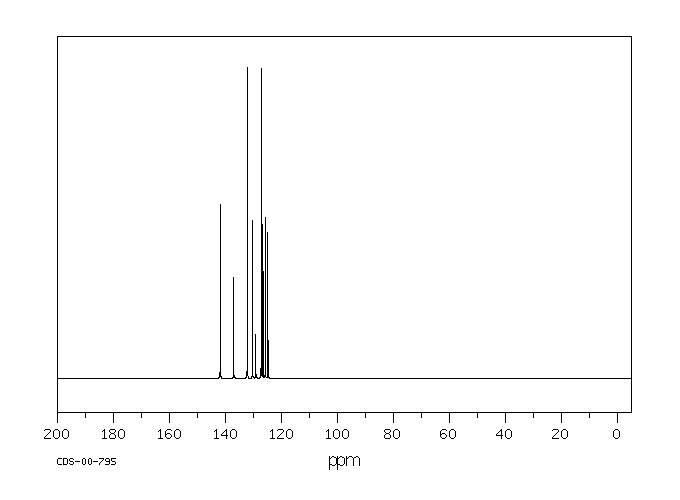
碳谱13CNMR
-
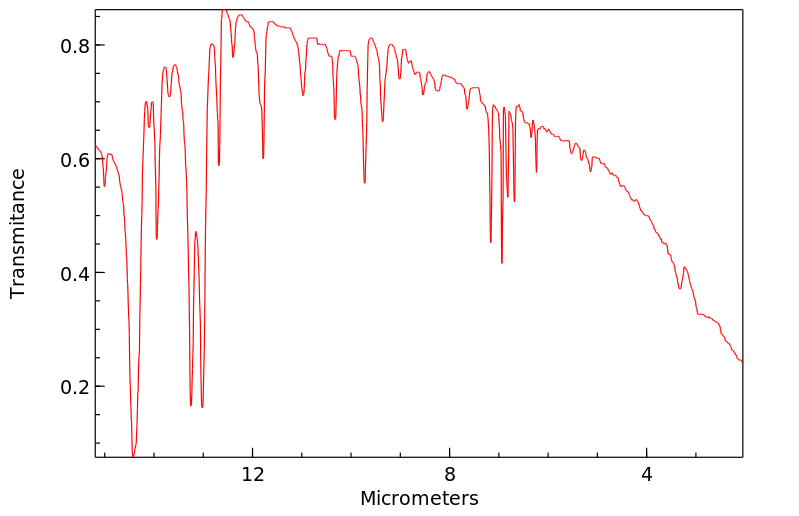
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2R,3R)-4-(蒽-9-基)-3-(叔丁基)-2-甲基-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
金不换萘酚
金不换素
蒽,9,10-二[4-(2,2-二苯基乙烯基)苯基]-
萘并[2,3-c]呋喃-1,3-二酮,6-甲氧基-4-(4-甲氧苯基)-
萘并[2,3-c]呋喃-1(3H)-酮,7-羟基-4-(3-甲氧苯基)-
萘并[2,3-c]呋喃-1(3H)-酮,4-(2-氟苯基)-7-(苯基甲氧基)-
萘,1-氯-2-乙基-3-甲基-4-苯基-
苯氧基-9苯基-10蒽
苯基-(10-苯基蒽-9-基)甲酮
红荧烯
甲基7-苯基二苯并(A,J)蒽-14-羧酸酯
甲基10-苯基-9-蒽羧酸酯
爵床脂素 B
爵床脂素 A
木酚素J1(P)
昔土米霉素
新爵床素 B
拒食胺
大麻酰胺
地蒽酚10,10'-二聚体
四去氢鬼臼毒素
叶下珠醇抑制剂A
双(4-(蒽-9-基)苯基)甲酮
二甲基4-(3,4-二甲氧苯基)-1-羟基-5,6,7-三甲氧基萘-2,3-二甲酸基酯
二叶草素
乙酸-(2-甲基-3,4-二苯基-[1]萘基酯)
[4-(3,10-二羟基蒽-9-基)苯基]乙酸乙酸酯
[4-(10-羟基蒽-9-基)苯基]乙酸乙酸酯
[2-甲氧基-10-(4-甲氧基苯基)蒽-9-基]乙酸酯
[10-羟基-5-(10-羟基-7,9-二甲氧基-3-甲基-3,4-二氢-1H-苯并[g]异苯并吡喃-5-基)-7,9-二甲氧基-3-甲基-3,4-二氢-1H-苯并[g]异苯并吡喃-4-基]乙酸酯
[10-(9,9-二甲基芴-2-基)蒽-9-基]硼酸
[10-(4-叔丁基苯基)蒽-9-基]硼酸
[(9,10-二苯基-[2]蒽基)-苯基-亚甲基]-琥珀酸
B-[10-(4-苯基-1-萘基)-9-蒽基]硼酸
B-(9,10-二苯基-2-蒽)硼酸
9.10-二(3',5'-二羧基苯基)蒽
9-萘-1-基-10-(4-苯基苯基)蒽
9-苯基蒽
9-苯基-10-苯乙炔基菲
9-苯基-10-硝基蒽
9-苯基-10-(苯基乙炔基)蒽
9-苯基-10-(4-三苯胺)蒽
9-苯基-1,2,3,4-四氢蒽
9-苄基-10-苯基蒽
9-羟基-10-甲氧基-5-(3,4,5-三甲氧基苯基)-8H-[2]苯并呋喃并[6,5-f][1,3]苯并二氧戊环-6-酮
9-碘-10-(10-碘蒽-9-基)蒽
9-甲氧基甲基-10-苯基蒽
9-甲氧基-10-苯基蒽
9-甲基-10-苯基菲