4-羟基哒嗪 | 20733-10-2
中文名称
4-羟基哒嗪
中文别名
——
英文名称
4-Hydroxypyridazin
英文别名
pyridazin-4-ol;4-Hydroxypyridazine;1H-pyridazin-4-one
CAS
20733-10-2
化学式
C4H4N2O
mdl
MFCD00233967
分子量
96.0886
InChiKey
VHVUTJZQFZBIRR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:315.9±15.0 °C(Predicted)
-
密度:1.293±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-0.1
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:41.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2933990090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,4-Dihydro-1-methyl-4-oxo-pyridazin 73619-53-1 C5H6N2O 110.115 5-氯-4-羟基哒嗪 4-chloro-5-hydroxypyridazin 1245643-70-2 C4H3ClN2O 130.534
反应信息
-
作为反应物:参考文献:名称:一种4,5-二羟基哒嗪的合成方法摘要:本发明属于医药化合物的合成技术领域,具体公开了一种4,5‑二羟基哒嗪的合成方法。本发明采用3,4,6‑三氯哒嗪为原料,先与氢氧化钠水溶液反应得到化合物2;然后化合物2用钯碳氢化脱掉氯得到化合物3;化合物3与N‑氯代丁二酰亚胺反应得到化合物4;之后化合物4与氢氧化钠水溶液反应得到最终产品4,5‑二羟基哒嗪。本发明方法具有原料便宜易得,生产方便,易于提纯的优点,可开发为工业化生产方法。公开号:CN113698355B
-
作为产物:描述:参考文献:名称:杂环研究中的化学研究。16. Mitteilung。哒嗪。3,4,6-三氯哒嗪取代摘要:3,4,6-三氯哒嗪被转化为许多新化合物,其结构由其化学和光谱性质确定。DOI:10.1002/hlca.19560390634
文献信息
-
具有吲哚胺2,3-双加氧酶抑制活性的稠合咪 唑化合物
-
AKR1C3抑制剂及医药用途申请人:深圳艾欣达伟医药科技有限公司公开号:CN112004799B公开(公告)日:2023-05-02本发明提供下式化合物及其药学上可接受的盐或溶剂合物或同位素取代化合物的AKR1C3抑制剂及医药用途
-
[EN] HERBICIDAL PYRIDAZINUM BASED COMPOUNDS<br/>[FR] COMPOSÉS HERBICIDES À BASE DE PYRIDAZINUM
-
Heilmittelchemische Studien in der heterocyclischen Reihe. 23. Mitteilung. Pyridazine XI. Die Reaktion von Kojisäure mit Hydrazin. 1. Teil作者:A. F. Thomas、A. MarxerDOI:10.1002/hlca.19580410645日期:——It has been shown that the reaction between kojic acid and hydrazine hydrate gives rise to two main products: 3,6-dihydroxymethyl-4-oxo-1,4-dihydro-pyridazine, and the hydrazone of 3-hydroxymethyl-pyrazol-5-yl-hydroxyacetaldehyde, the structures of which were proved unambiguously.
-
Direct C–H Hydroxylation of N-Heteroarenes and Benzenes via Base-Catalyzed Halogen Transfer作者:Kendelyn I. Bone、Thomas R. Puleo、Jeffrey S. BandarDOI:10.1021/jacs.3c14058日期:2024.4.10employs an alkoxide base to catalyze X-transfer from sacrificial 2-halothiophene oxidants to aryl substrates, forming SNAr-active intermediates that undergo nucleophilic hydroxylation. Key to this process is the use of 2-phenylethanol as an inexpensive hydroxide surrogate that, after aromatic substitution and rapid elimination, provides the hydroxylated arene and styrene byproduct. Use of simple 2-halothiophenes羟基化(杂)芳烃在许多行业中都受到重视,既是最终产品的关键成分,又是可多样化的合成结构单元。因此,开发补充和解决引入芳香族羟基的现有方法的局限性的反应是一个重要的目标。为此,我们应用碱催化的卤素转移(X-转移)来实现弱酸性N-杂芳烃和苯的直接C-H羟基化。该方案采用醇盐碱催化从牺牲的 2-卤代噻吩氧化剂到芳基底物的 X 转移,形成发生亲核羟基化的 S N Ar 活性中间体。该过程的关键是使用 2-苯基乙醇作为廉价的氢氧化物替代物,在芳族取代和快速消除后,提供羟基化芳烃和苯乙烯副产物。使用简单的2-卤代噻吩可以实现6元N-杂芳烃和1,3-唑衍生物的C-H羟基化,而合理设计的2-卤代苯并噻吩氧化剂则将范围扩展到缺电子苯底物。机理研究表明芳香族 X 转移是可逆的,这表明去质子化、卤化和取代步骤协同作用,表现出独特的选择性趋势,不一定依赖于酸性最强的芳基位置。通过简化的目标分子合成、与替代 C-H
表征谱图
-
氢谱1HNMR
-
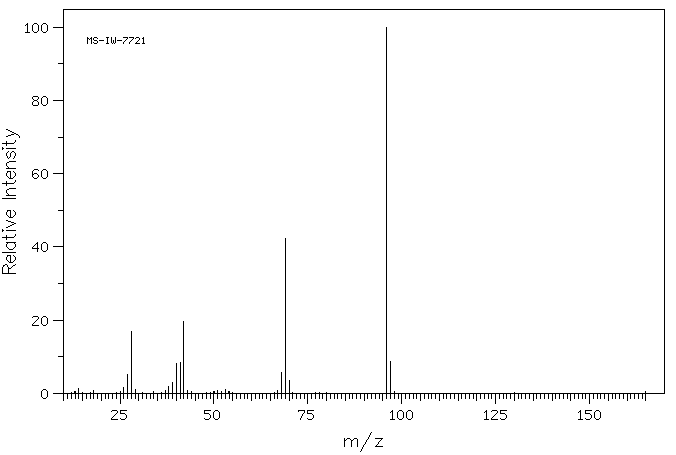
质谱MS
-
碳谱13CNMR
-
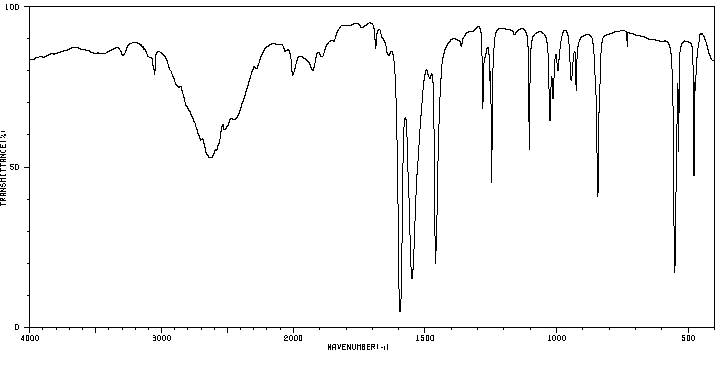
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3