4-苯基-1,3-二硫杂-2-硫代-环戊-4-烯 | 2314-61-6
中文名称
4-苯基-1,3-二硫杂-2-硫代-环戊-4-烯
中文别名
——
英文名称
4-phenyl-1,3-dithiole-2-thione
英文别名
4-phenyl-1,3-dithia-2-thioxo-cyclopent-4-ene;phenyl-4 dithiole-1,3 thiones-2;4-phenyl-2-thioxo-1,3-dithiole;4-phenyl-1,3-dithiol-2-thione;4-phenyl-[1,3]dithiole-2-thione;3-Phenyl-2,5-dithia-cyclopenten-(3)-thion-(1)
CAS
2314-61-6
化学式
C9H6S3
mdl
——
分子量
210.345
InChiKey
ZLHIKMZHVHFMJK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:82.7
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4-phenyl-1,3-dithiol-2-one 939-11-7 C9H6OS2 194.278
反应信息
-
作为反应物:描述:4-苯基-1,3-二硫杂-2-硫代-环戊-4-烯 在 dicobalt octacarbonyl 作用下, 以 甲苯 为溶剂, 反应 1.5h, 以25%的产率得到4,4'-diphenyl tetrathiafulvalene参考文献:名称:Coustumer, Gerard Le; Mollier, Yves, Journal of the Chemical Society. Chemical communications, 1980, # 1, p. 38 - 39摘要:DOI:
-
作为产物:参考文献:名称:Efficient and general synthesis of 1,3-dithiole-2-thiones摘要:DOI:10.1021/jo01289a041
文献信息
-
Titanium(salen)-Catalysed Synthesis of Di- and Trithiocarbonates from Epoxides and Carbon Disulfide作者:Christopher Beattie、Michael NorthDOI:10.1002/cctc.201400005日期:2014.3.12tributylamine forms a highly active catalyst system for the reaction between epoxides and carbon disulfide to lead to di‐ and/or trithiocarbonates. Reactions can be performed at 90 °C by using just 0.5–1.0 mol % of the catalysts. The reactions proceed with the inversion of the epoxide configuration and on the basis of kinetic and spectroscopic evidence, a mechanism to account for the results is proposed.
-
A Convenient Preparation of 1,3-Dithiole-2-thione and 1,3-Diselenole-2-selone Derivatives作者:K. Takimiya、A. Morikami、T. OtsuboDOI:10.1055/s-1997-787日期:1997.3A convenient one-pot preparation of 1,3-dithiole-2-thiones and 1,3-diselenole-2-selones substituted with phenyl, alkyl, alkylthio, hydroxymethyl, and formyl groups was accomplished from readily available acetylenes in good to excellent yields.
-
A Simple Synthesis of 2-Methyl-1,3-Dithiolium and Related Cations作者:Javier Garín、Raquel Andreu、Laura Carrasquer、Miguel Cerdán、Ana Fernández、Santiago Franco、Jesús OrdunaDOI:10.1055/s-2007-980362日期:2007.6A simple, high-yielding synthesis of 2-methyl-1,3-dithi- olium tetrafluoroborates is reported. The reaction of these salts with N,N-dimethylformamide and acetic anhydride gives rise to the pre- viously unreported N,N-dimethyl 2-(1,3-dithiol-2-yliden)ethyliden- iminium tetrafluoroborates.
-
Synthesis and electrochemical studies of tetrathiafulvalene derivatives (TTFs) as redox active ligands作者:Abd El-Wareth A. O. Sarhan、Makoto Murakami、Taeko IzumiDOI:10.1002/jhet.5570390413日期:2002.7simple route for synthesis of new tetrathiafulvalene dimethyl ester (TTF-DME) is reported. The tetrathifulvalene dimethylester (TTF-DME) has been prepared by introducing an ester coordination function as a bifunctional new donor. The redox behavior of the TTF-DME was investigated in comparison to the well-known dibenzotetrathiafulvene (DB-TTF) by cyclic voltammetry. A two-electron redox behavior was observed
-
Thermolysis of 1, 3-dithiol-2-yl azides and thermal properties of the resulting 1,4,2-dithiazines作者:Juzo Nakayama、Atsuhiro Sakai、Akira Tokiyama、Masamatsu HoshinoDOI:10.1016/s0040-4039(00)94520-9日期:1983.1azides, on thermolysis in refluxing toluene, give 3-substituted 1,4,2-dithiazines which thermally extrude sulfur atom of the 4-position selectively to yield 3-substituted isothiazoles, while 2-unsubstituted 1,3-dithiol-2-yl azides undergo both ring expansion yielding 1,4,2-dithiazines and peculiar thermal dissociation into 1,3-dithiol-2-ylidene carbenes and hydrogen azide.
表征谱图
-
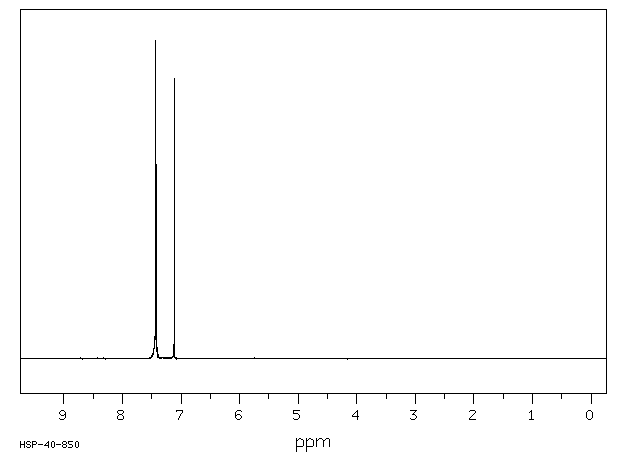
氢谱1HNMR
-
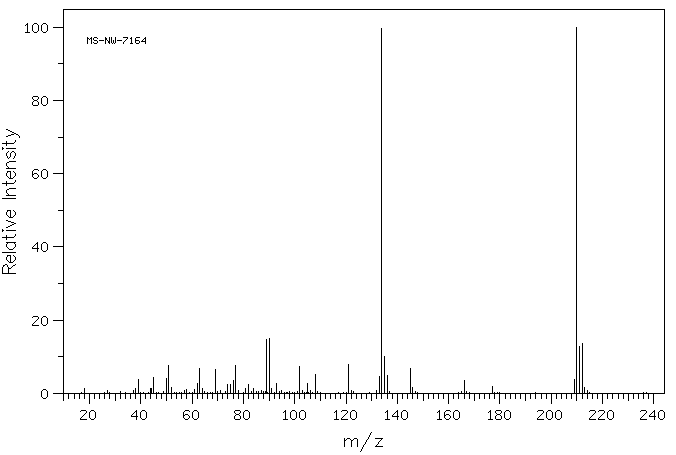
质谱MS
-
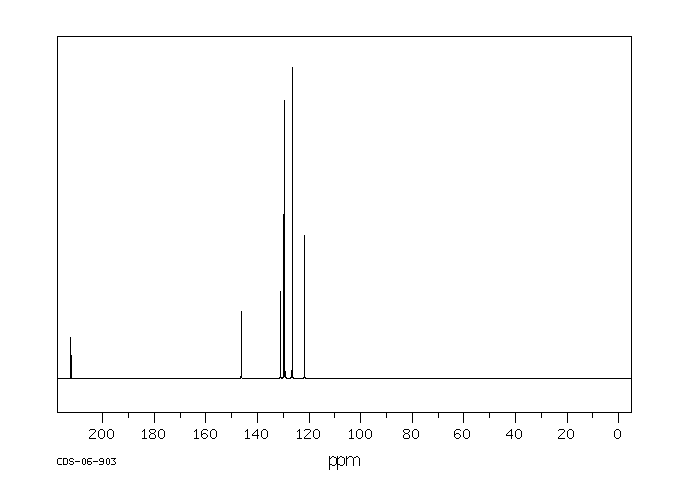
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫