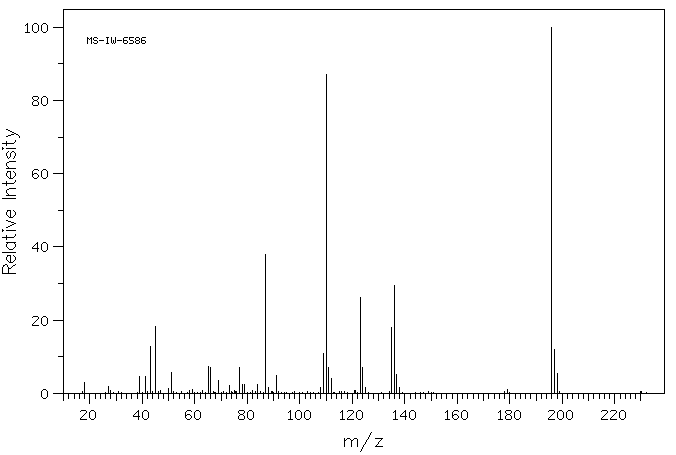
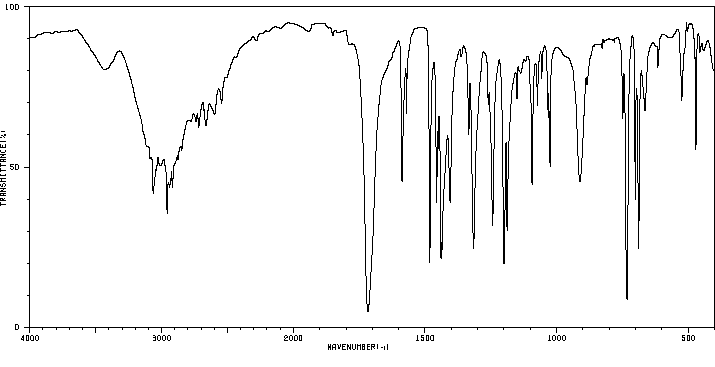
4-苯氨磺酰基丁酸 | 17742-51-7
中文名称
4-苯氨磺酰基丁酸
中文别名
——
英文名称
4-phenylsulfanyl-butyric acid
英文别名
γ-Phenylmercapto-buttersaeure;4-(phenylthio)butyric acid;4-(phenylthio)butanoic acid;phenylthiobutanoic acid;PTBA;4-(Phenylsulfanyl)butanoic acid;4-phenylsulfanylbutanoic acid
CAS
17742-51-7
化学式
C10H12O2S
mdl
MFCD02082831
分子量
196.27
InChiKey
HHZVQLOVHIDMBD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:68-69 °C(Solv: ligroine (8032-32-4))
-
沸点:362.9±25.0 °C(Predicted)
-
密度:1.19±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:13
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:62.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2930909090
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:应存放在室温、干燥且密封的环境中。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 4-(phenylthio)butanoate 29193-71-3 C11H14O2S 210.297 —— γ-Phenylmercapto-α-carboxy-buttersaeure 1020-68-4 C11H12O4S 240.28 4-苯基巯基丁腈 4-(phenylthio)butanenitrile 35756-39-9 C10H11NS 177.27 3-(苯基硫代)-1-丙醇 3-phenylthiopropanol 24536-40-1 C9H12OS 168.26 [(3-溴丙基)硫基]苯 3-phenylthio-1-bromopropane 3238-98-0 C9H11BrS 231.156 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 4-(phenylthio)butanoate 29193-71-3 C11H14O2S 210.297 —— ethyl 4-(phenylthio)butanoate 29193-72-4 C12H16O2S 224.324 —— Butyl 4-phenylsulfanylbutanoate 29193-75-7 C14H20O2S 252.378 —— 4-Phenylsulfanyl-butyric acid isopropyl ester 29193-74-6 C13H18O2S 238.351 —— (R)-(+)-4-(phenylthio)-2-methyl-1-butanol 129518-67-8 C11H16OS 196.313 —— 4-phenylsulfanyl-butyryl chloride 138738-23-5 C10H11ClOS 214.716 —— methyl 4-[(4-chlorophenyl)thio]butanoate 29107-78-6 C11H13ClO2S 244.742 —— 4-(phenylsulfanyl)butan-1-amine 50554-93-3 C10H15NS 181.302 —— 4-(phenylsulfinyl)butyric acid 49639-32-9 C10H12O3S 212.269 4-((4-氯苯基)硫代)丁酸乙酯 ethyl 4-(4-chlorphenylthio)butanoate 29107-85-5 C12H15ClO2S 258.769 —— Propyl 4-[(4-chlorophenyl)sulfanyl]butanoate 29107-86-6 C13H17ClO2S 272.796 N-羟基-4-(苯基硫代)丁酰胺 N-hydroxy-4-(phenylthio)butanamide 10376-60-0 C10H13NO2S 211.285 —— Butyl 4-(4-chlorophenyl)sulfanylbutanoate 29107-88-8 C14H19ClO2S 286.823 —— Propan-2-yl 4-(4-chlorophenyl)sulfanylbutanoate 29107-87-7 C13H17ClO2S 272.796 —— N-<4-(Phenylthio)butyl>acetamide 130422-34-3 C12H17NOS 223.339 4-苯磺酰基丁酸 4-(phenylsulfonyl)butanoic acid 6178-52-5 C10H12O4S 228.269 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Traynelis; Love, Chemistry and industry, 1958, p. 439摘要:DOI:
-
作为产物:参考文献:名称:金催化炔基芳基亚砜转化中的 [3,3]-Sigmatropic 重排与卡宾形成:机理研究和扩展反应范围摘要:以芳基亚磺酰基为束缚氧化剂的末端炔烃的金催化分子内氧化是金化学中具有重大影响的反应,因为它向该领域引入了通过炔氧化生成金卡宾的高度有价值的概念。然而,α-氧代金卡宾在这些反应中的中介作用从未得到证实。详细的实验研究表明,此类反应性中间体参与二氢苯并硫杂环己烷的形成的可能性极小。相反,初始环化中间体的[3,3]-σ重排提供了一条反应路径,可以很容易地解释高反应效率和缺乏锍形成。然而,对于内部炔烃底物,金卡宾物质的生成变得与[3,3]-σ重排竞争。然而,这种反应性中间体不会继续提供弗里德尔-克拉夫茨型环化产物。广泛的密度泛函理论研究支持了这样的机制结论:环化产物是通过分子内[3,3]-σ重排形成的,而不是之前提出的弗里德尔-克拉夫茨型环化。凭借新的机理见解,这种多功能形成的中型含硫环烯酮的产品范围已轻松扩展到各种二氢苯并硫辛酮、四氢苯并环壬酮,甚至那些没有稠合苯环的化合物。除了金之外,Hg(OTf)2DOI:10.1021/ja401343p
文献信息
-
Piperazine and homopiperazine compounds
-
Substituted (aryl, heteroaryl, arylmethyl or heteroarylmethyl)申请人:Rhone-Poulenc Rorer Pharmaceuticals Inc.公开号:US06057369A1公开(公告)日:2000-05-02This invention is directed to compounds of formula I: ##STR1## wherein the variables are as described herein. Compounds within the scope of the present invention possess useful properties, more particularly pharmaceutical properties. They are especially useful for inhibiting the production or physiological effects of TNF in the treatment of a patient suffering from a disease state associated with a physiologically detrimental excess of tumor necrosis factor (TNF). Compounds within the scope of the present invention also inhibit cyclic AMP phosphodiesterase, and are useful in treating a disease state associated with pathological conditions that are modulated by inhibiting cyclic AMP phosphodiesterase, such disease states including inflammatory and autoimmune diseases, in particular type IV cyclic AMP phosphodiesterase. Compounds within the scope of the present invention may also inhibit an MMP, and are useful in treating a disease state associated with pathological conditions that are modulated by inhibiting MMPs, such disease states involve tissue breakdown and those associated with a physiologically detrimental excess of TNF. The present invention is therefore also directed to the pharmaceutical use of the compounds, pharmaceutical compositions containing the compounds, intermediates leading thereto and methods for the preparation of the compounds and their intermediates.这项发明涉及到式I的化合物:##STR1## 其中变量如本文所述。本发明范围内的化合物具有有用的性质,更具体地说是药用性质。它们特别适用于抑制TNF的产生或生理效应,用于治疗患有与生理上有害的肿瘤坏死因子(TNF)过量相关的疾病状态的患者。本发明范围内的化合物还抑制环状AMP磷酸二酯酶,并且适用于治疗与通过抑制环状AMP磷酸二酯酶调节的病理条件相关的疾病状态,这些疾病状态包括炎症和自身免疫疾病,特别是第四型环状AMP磷酸二酯酶。本发明范围内的化合物还可能抑制MMP,并且适用于治疗与通过抑制MMPs调节的病理条件相关的疾病状态,这些疾病状态涉及组织分解以及与生理上有害的TNF过量相关的疾病状态。因此,本发明还涉及化合物的药用,含有该化合物的药物组合物,导致该化合物的中间体以及该化合物及其中间体的制备方法。
-
Neighboring Group Participation in the Halogenation of Sulfoxides作者:T. Durst、K.-C. Tin、M. J. V. MarcilDOI:10.1139/v73-256日期:1973.6.1
PhS(O)(CH2)nCH2OH(n = 1–3) react with sulfuryl chloride at −78° or 0° in methylene chloride to give the corresponding PhSO2(CH2)nCH2Cl. Evidence is presented that cyclic alkoxyoxosulfonium salts are intermediates in these transformations. When n = 4 only chlorination α to the sulfoxide function was observed. The sulfinyl carboxylic acids PhS(O)(CH2)nCO2H (n = 1–3) and amides PhS(O)(CH2)nCONH2 (n = 1–3) were also reacted with SO2Cl2. α-Chlorination was observed for both compounds when n = 1 or 3. When n = 2 the products were the 3-phenylsulfonylpropionyl chloride and 3-phenyl-sulfonylpropionitrile respectively.
-
Synthesis of a Wide Range of Thioethers by Indium Triiodide Catalyzed Direct Coupling between Alkyl Acetates and Thiosilanes作者:Yoshihiro Nishimoto、Aya Okita、Makoto Yasuda、Akio BabaDOI:10.1021/ol300450j日期:2012.4.6An indium triiodide-catalyzed substitution of the acetoxy group in alkyl acetates with thiosilanes provides access to a variety of thioethers. The method is efficient for a wide scope of acetates such as primary alkyl, secondary alkyl, tertiary alkyl, allylic, benzylic, and propargylic acetates.
-
Über die Eigenschaften von (Arylmercapto)acethydroxamsäuren作者:S. M. A. D. Zayed、I. Y. Mostafa、M. FarghalyDOI:10.1002/ardp.19673000608日期:——Die Darstellung einiger gegen phytopathogene Pilze wirksamer, substituierter Mercaptoacethydroxamsäuren sowie (Phenylmercapto)butyr‐ und (Phenylmercapto)valerylhydroxamsäuren wird beschrieben, die IR‐Spektren einiger (Phenylmercapto)acet‐und (Phenylsulfonyl)acethydroxamsäuren werden diskutiert.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯