9-benzylanthracene | 1498-71-1
中文名称
——
中文别名
——
英文名称
9-benzylanthracene
英文别名
9-Benzyl-anthracen
CAS
1498-71-1
化学式
C21H16
mdl
——
分子量
268.358
InChiKey
OMKKTUHEEREHJB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:133 °C
-
沸点:452.0±15.0 °C(Predicted)
-
密度:1.131±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):6.3
-
重原子数:21
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯基(9-蒽基)甲酮 9-benzoylanthracene 1564-53-0 C21H14O 282.342 —— 9-(1-hydroxybenzyl)-10-methylanthracene 72948-52-8 C21H16O 284.357 9-蒽醇 9-hydroxymethylanthracene 1468-95-7 C15H12O 208.26 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 9-benzyl-10-chloromethylanthracene 24451-71-6 C22H17Cl 316.83
反应信息
-
作为反应物:描述:参考文献:名称:Cook, Journal of the Chemical Society, 1926, p. 2171摘要:DOI:
-
作为产物:描述:参考文献:名称:环庚三烯,奥氮平,硫平和西乐平的合成:布朗斯台德酸和金催化的比较摘要:遵循以下不同的路径:描述了在布朗斯台德酸和金(I)催化下1-(苄基,氧芳基,硫代芳基和甲基芳基)-2-乙炔基苯的环化。尽管Au催化优先提供7元环,但酸促进的环化反应会导致蒽,吡喃鎓盐和S-(芳基)苯并噻吩鎓盐的形成。DOI:10.1002/ejoc.202001072
文献信息
-
Cross-coupling reactions with boronic acids in water catalysed by oxime-derived palladacycles作者:Luis Botella、Carmen NájeraDOI:10.1016/s0022-328x(02)01727-8日期:2002.12Palladacycles derived from phenone-oximes 1 are efficient precatalysts for the Suzuki–Miyaura coupling of arylboronic acids with aromatic and heteroaromatic bromides and chlorides under water reflux under aerobic conditions. Alternatively, the coupling can also be carried out at room temperature in methanol–water. Aryl bromides gave biaryls with TON up to 105 and TOF up to 7×104 h−1. Activated and
-
A Palladium Bipyridyl Complex Grafted onto Nanosized MCM-41 as a Heterogeneous Catalyst for Negishi Coupling作者:Wei-Yi Wu、Tze-Chiao Lin、Tamotsu Takahashi、Fu-Yu Tsai、Chung-Yuan MouDOI:10.1002/cctc.201200388日期:2013.4The Negishi coupling of aryl bromides or acyl chlorides with organozinc chlorides catalyzed by a palladium bipyridyl complex anchored on nanosized mobile crystalline material 41 (MCM‐41) were investigated. The reactions proceeded smoothly with a very low catalyst loading in THF at 70 °C for electron‐deficient aryl bromides, which gave good to high yields of the Negishi coupling products. However, reactions
-
Alumina grafted SBA-15 sustainable bifunctional catalysts for direct cross-coupling of benzylic alcohols to diarylmethanes作者:Chandran Rajendran、Govindaswamy Satishkumar、Charlotte Lang、Eric M. GaigneauxDOI:10.1039/d0cy00471e日期:——desorption (TPD) and pyridine-transmission-FTIR spectroscopy techniques to confirm the existence of Brønsted acid and Lewis acid–base bifunctionalities. Through various control experiments, it is verified that Brønsted acid sites activate the benzyl alcohol and Lewis base sites interact with phenylboronic acid concurrently to accomplish the coupling reaction. In the recyclability study, 4wt%AlSBA-15 preserves具有布朗斯台德酸和路易斯酸碱双官能度的AlSBA-15催化剂可催化苄醇直接芳基化为二芳基甲烷,并通过C-O键活化获得85%的产率。通过采用简单有效的合成后金属注入途径,合成了2wt%和4wt%AlSBA-15催化剂。用XRD,N 2吸附和解吸,27 Al MAS NMR,XPS,HR-TEM,NH 3和CO 2表征合成的催化剂。程序升温脱附(TPD)和吡啶传递FTIR光谱技术确定了布朗斯台德酸和路易斯酸碱双官能团的存在。通过各种控制实验,证实布朗斯台德酸位点激活苯甲醇,路易斯碱位点同时与苯硼酸相互作用,完成偶联反应。在可回收性研究中,4wt%AlSBA-15最多可保留5个循环的活性和稳定性。与均相催化剂不同,4wt%AlSBA-15催化剂不需要添加剂,较长的反应时间和昂贵的金属。
-
Reactions of cupric halides with organic compounds—III作者:D. Mosnaim、D.C. Nonhebel、J.A. RussellDOI:10.1016/s0040-4020(01)82883-0日期:1969.19-Alkyl and 9-arylanthracenes have been shown to undergo halogenation with cupric halides to give good yields of their 10-halogenated derivatives. Electron-donating groups in 9-arylanthracenes were shown to increase the rate of reaction. The results are interpreted in terms of a ligand-transfer mechanism.
-
Direct Arylation/Alkylation/Magnesiation of Benzyl Alcohols in the Presence of Grignard Reagents via Ni-, Fe-, or Co-Catalyzed sp<sup>3</sup> C–O Bond Activation作者:Da-Gang Yu、Xin Wang、Ru-Yi Zhu、Shuang Luo、Xiao-Bo Zhang、Bi-Qin Wang、Lei Wang、Zhang-Jie ShiDOI:10.1021/ja307045r日期:2012.9.12Direct application of benzyl alcohols (or their magnesium salts) as electrophiles in various reactions with Grignard reagents has been developed via transition metal-catalyzed sp(3) C-O bond activation. Ni complex was found to be an efficient catalyst for the first direct cross coupling of benzyl alcohols with aryl/alkyl Grignard reagents, while Fe, Co, or Ni catalysts could promote the unprecedented
表征谱图
-
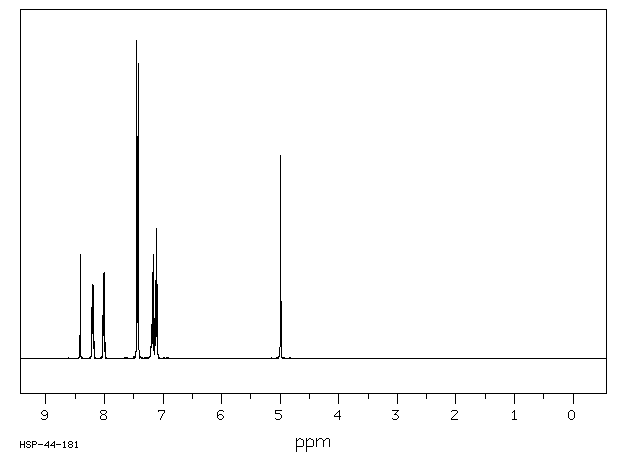
氢谱1HNMR
-
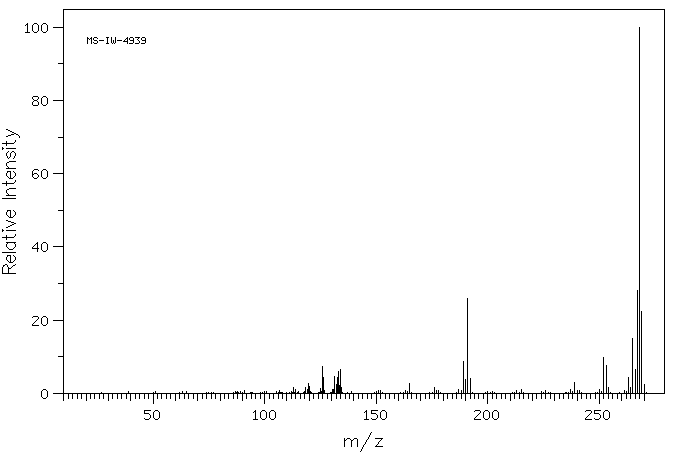
质谱MS
-
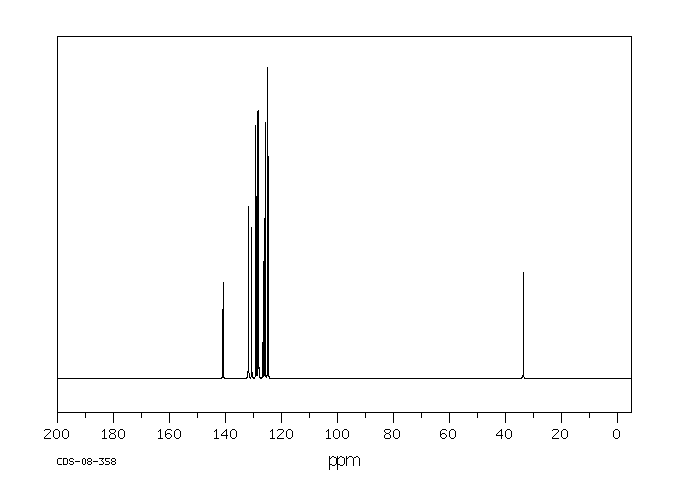
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62