cyclohexanone oxime acetate | 19689-92-0
中文名称
——
中文别名
——
英文名称
cyclohexanone oxime acetate
英文别名
cyclohexanone O-acetyl oxime;Cyclohexanone, oxime, acetate;(cyclohexylideneamino) acetate
CAS
19689-92-0
化学式
C8H13NO2
mdl
——
分子量
155.197
InChiKey
PRUSJJMORDRYMN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:228.4±23.0 °C(Predicted)
-
密度:0.9870 g/cm3
-
保留指数:1148
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:38.7
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:参考文献:名称:Reduction of O-Acyl Oximes with Sodium Borohydride/Iodine System摘要:O-acyl derivatives of aldoximes and ketoximes are reduced in good yields to the corresponding amines with sodium borohydride-iodine system.DOI:10.1080/00397919508015484
-
作为产物:描述:参考文献:名称:通过4-羟基香豆素和酮肟之间的铜催化环化反应 获得取代的呋喃香豆素的新途径†摘要:开发了一种通过4-羟基香豆素和酮肟之间的铜催化环化反应取代呋喃香豆素的新途径。CuBr 2表现出比其他铜盐更高的活性,从而以高收率提供了所需的呋喃香豆素。在没有化学计量外部氧化剂的情况下,转化容易进行。这种综合策略的意义将是:(1)容易获得的起始材料;(2)低成本催化剂CuBr 2;(3)没有化学计量的外部氧化剂。该协议是取代呋喃香豆素合成中先前方法的补充。DOI:10.1039/c8ob01064a
文献信息
-
Iron-Catalyzed Synthesis of 2<i>H</i>-Imidazoles from Oxime Acetates and Vinyl Azides under Redox-Neutral Conditions作者:Zhongzhi Zhu、Xiaodong Tang、Jianxiao Li、Xianwei Li、Wanqing Wu、Guohua Deng、Huanfeng JiangDOI:10.1021/acs.orglett.7b00203日期:2017.3.17A novel and versatile method for the synthesis of 2H-imidazoles via iron-catalyzed [3 + 2] annulation from readily available oxime acetates with vinyl azides has been developed. This denitrogenative process involved N–O/N–N bond cleavages and two C–N bond formations to furnish 2,4-substituted 2H-imidazoles. This protocol was performed under mild reaction conditions and needed no additives or ligands
-
Copper-catalyzed synthesis of thiazol-2-yl ethers from oxime acetates and xanthates under redox-neutral conditions作者:Zhongzhi Zhu、Xiaodong Tang、Jinghe Cen、Jianxiao Li、Wanqing Wu、Huanfeng JiangDOI:10.1039/c8cc00445e日期:——acetates and xanthates for the synthesis of thiazol-2-yl ethers with remarkable regioselectivity has been developed. Various oxime acetates, whether derived from aryl ketones or alkyl ketones, or natural product cores are suitable for this conversion. Unique dihydrothiazoles were also obtained when both reaction sites were methine. Mechanistic studies indicated that imino copper(III) intermediates were involved
-
Divergent Iron-Catalyzed Coupling of<i>O</i>-Acyloximes with Silyl Enol Ethers作者:Hai-Bin Yang、Nicklas SelanderDOI:10.1002/chem.201605636日期:2017.2.3An iron‐catalyzed coupling reaction of O‐acyloximes and O‐benzoyl amidoximes with silyl enol ethers is reported. The protocol provides access to functionalized pyrroles, 1,6‐ketonitriles, pyrrolines and imidazolines via carbon‐centered radicals generated from an initially formed iminyl radical. The intramolecular cyclization and ring‐opening processes of the iminyl radical take place preferentially
-
Catalyst free synthesis of mono- and disubstituted pyrimidines from O-acyl oximes作者:Atul Upare、Pochampalli Sathyanarayana、Ranjith Kore、Komal Sharma、Surendar Reddy BathulaDOI:10.1016/j.tetlet.2018.05.023日期:2018.6catalyzed transformations of O-acyl oximes to various N-heterocycles are well established. Herein, we report a catalyst free, oxime carboxylate based, three-component condensation method to access mono- and disubstituted pyrimidines. A broad range of substituted pyrimidines were prepared in moderate to excellent yields. Control experiments reveal that in situ generated formamidine is the key intermediate.
-
Copper-catalyzed radical/radical cross-coupling of ketoxime carboxylates with 4-hydroxycoumarins: A novel synthesis of furo[3,2-c]-coumarins作者:Mingchuang He、Zhaohua Yan、Wangyang Wang、Fuyuan Zhu、Sen LinDOI:10.1016/j.tetlet.2018.09.007日期:2018.10A novel and efficient strategy for the synthesis of furo[3,2-c]coumarins has been developed via copper-catalyzed radical/radical cross-coupling of ketoxime carboxylates with 4-hydroxycoumarins. This redox-neutral reaction allows smooth and selective synthesis of 2-substituted, 3-substituted, 2,3-disubstituted and 2,3-fused polycyclic furo[3,2-c]coumarins.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
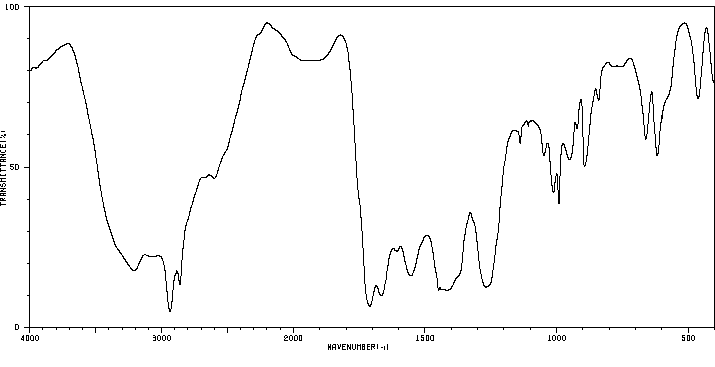
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸