N-([1,1'-biphenyl]-4-yl)-4-methylbenzenesulfonamide | 65690-69-9
中文名称
——
中文别名
——
英文名称
N-([1,1'-biphenyl]-4-yl)-4-methylbenzenesulfonamide
英文别名
N-[1,1'-Biphenyl]-4-yl-4-methylbenzenesulfonamide;4-methyl-N-(4-phenylphenyl)benzenesulfonamide
CAS
65690-69-9
化学式
C19H17NO2S
mdl
——
分子量
323.415
InChiKey
VOJVNWBUZFVCEJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:492.8±48.0 °C(Predicted)
-
密度:1.243±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:23
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:54.6
-
氢给体数:1
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-羟基-4-甲基-N-苯基苯磺酰胺 N-phenyl N-(4-methylphenylsulfonyl) hydroxylamine 38557-76-5 C13H13NO3S 263.317
反应信息
-
作为反应物:参考文献:名称:Bell, Journal of the Chemical Society, 1928, p. 2778摘要:DOI:
-
作为产物:描述:参考文献:名称:Bell; Kenyon; Robinson, Journal of the Chemical Society, 1926, p. 1244摘要:DOI:
文献信息
-
A highly efficient heterogeneous copper-catalyzed Chan–Lam coupling reaction of sulfonyl azides with arylboronic acids leading to N-arylsulfonamides作者:Chongren You、Fang Yao、Tao Yan、Mingzhong CaiDOI:10.1039/c6ra04298h日期:——A heterogeneous Chan–Lam coupling reaction between sulfonyl azides and arylboronic acids was achieved in MeOH at room temperature in the presence of 10 mol% of an L-proline-functionalized MCM-41-immobilized copper(I) complex [MCM-41–L-proline–CuCl] under air, yielding a variety of N-arylsulfonamides in excellent yields. The new heterogeneous copper complex can be prepared from commercially readily
-
Synthesis of <i>N</i>-arylsulfonamides through a Pd-catalyzed reduction coupling reaction of nitroarenes with sodium arylsulfinates作者:Bo Yang、Chang Lian、Guanglu Yue、Danyang Liu、Liyan Wei、Yi Ding、Xiancai Zheng、Kui Lu、Di Qiu、Xia ZhaoDOI:10.1039/c8ob02226g日期:——A novel one-step direct reductive coupling reaction between nitroarenes and sodium arylsulfinates was realized in the presence of an inexpensive Pd/C catalyst. In this procedure, readily available nitroarenes are employed as the nitrogen sources, and sodium arylsulfinates serve as both coupling partners and reductants. The method features high efficiency by using cheap Pd/C with low catalyst loading
-
Direct CH Amidation of Benzoic Acids to Introduce<i>meta</i>- and<i>para</i>-Amino Groups by Tandem Decarboxylation作者:Donggun Lee、Sukbok ChangDOI:10.1002/chem.201500331日期:2015.3.27The Ir‐catalyzed mild CH amidation of benzoic acids with sulfonyl azides was developed to give reactions with high efficiency and functional‐group compatibility. Subsequent protodecarboxylation of ortho‐amidated benzoic acid products afforded meta‐ or para‐substituted (N‐sulfonyl)aniline derivatives, the latter being inaccessible by other CH functionalization approaches. The decarboxylation step
-
Accessing bridged bicyclic compounds or meta carbon-functionalized anilines from the dearomatization of anilines作者:Linfei Wang、Shuo-En Wang、Weibin Wang、Renhua FanDOI:10.1039/c3ra23224g日期:——The oxidative dearomatization of anilines was combined with a domino Michael addition, providing a series of nitrogen-containing bridged bicyclic compounds in moderate to excellent yields. The bridged bicyclic compound could be converted into the corresponding meta carbon-functionalized aniline derivative via a quinine-catalyzed tandem retro-oxa-Michael addition–aromatization reaction.
-
Selective C3C3 Oxidative Cross-Coupling between Unactivated Anilines and Indoles作者:Linfei Wang、Zhaomeng Han、Renhua FanDOI:10.1002/adsc.201000603日期:2010.12.17A selective C3C3 oxidative cross-coupling between unactivated anilines and indoles catalyzed by copper bromide together with iodobenzene diacetate as the oxidant is described. This methodology provides a novel approach to biaryl synthesis.
表征谱图
-
氢谱1HNMR
-
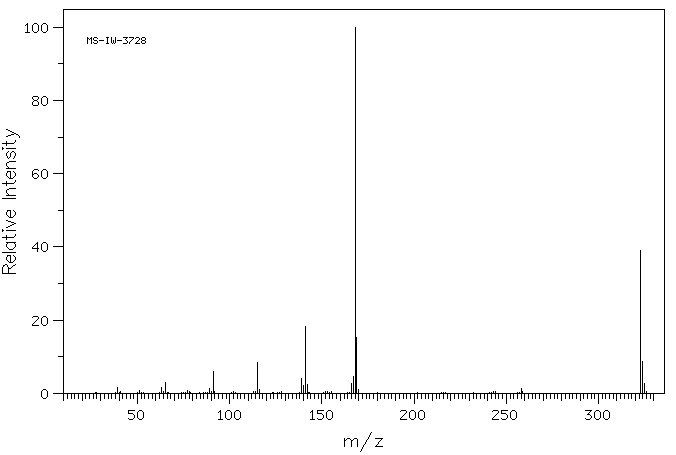
质谱MS
-
碳谱13CNMR
-
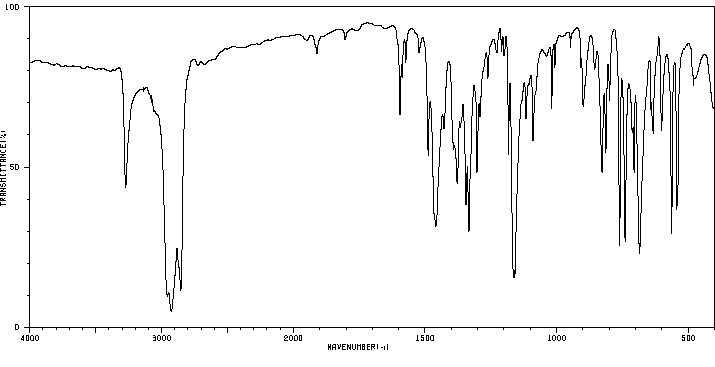
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫