Tetracyclin*HCl | 64-75-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:217 °C
-
溶解度:DMSO(微溶)、甲醇(微溶、超声处理)、水(微溶、超声处理)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):0.21
-
重原子数:33.0
-
可旋转键数:2.0
-
环数:4.0
-
sp3杂化的碳原子比例:0.41
-
拓扑面积:181.62
-
氢给体数:6.0
-
氢受体数:9.0
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:2
-
海关编码:29413000
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:QI9100000
-
危险类别:3
-
储存条件:存储在阴凉干燥的环境中。
SDS
| Name: | 4-Epitetracycline Hydrochloride "Can be used as Secondary Standard." Material Safety Data Sheet |
| Synonym: | Quatrimycin Hydrochloride |
| CAS: | 23313-80-6 |
Synonym:Quatrimycin Hydrochloride
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 23313-80-6 | 4-Epitetracycline Hydrochloride | ca 100 | unlisted |
Risk Phrases: 63
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Possible risk of harm to the unborn child.Light sensitive.Moisture sensitive.Air sensitive.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. May cause liver and kidney damage. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. May cause liver and kidney damage. The toxicological properties of this substance have not been fully investigated.
Chronic:
May cause liver and kidney damage. May cause reproductive and fetal effects.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Do NOT get water inside containers. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation. Place under an inert atmosphere. Do not get water inside containers.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation. Store protected from light. Handle under an inert atmosphere. Store protected from air. Do not allow contact with water. Keep from contact with moist air and steam.
Storage:
Do not store in direct sunlight. Store in a tightly closed container. Keep under a nitrogen blanket. Store in a cool, dry, well-ventilated area away from incompatible substances. Do not expose to air. Store protected from moisture. Store protected from light.
Store under an inert atmosphere.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 23313-80-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: yellow
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 217 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: > 217 deg C
Solubility in water: in water: 1:10 m/v
Specific Gravity/Density:
Molecular Formula: C22H24N2O8.HCl
Molecular Weight: 480.89
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, light, moisture, exposure to air, exposure to moist air or water.
Incompatibilities with Other Materials:
Moisture, air.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 23313-80-6: QJ1898250 LD50/LC50:
Not available.
Carcinogenicity:
4-Epitetracycline Hydrochloride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 63 Possible risk of harm to the unborn child.
Safety Phrases:
S 28A After contact with skin, wash immediately with
plenty of water.
S 36/37 Wear suitable protective clothing and
gloves.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 23313-80-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 23313-80-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 23313-80-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:描述:参考文献:名称:Constructing Nano‐Heterostructure with Dual‐Site to Boost H2O2 Activation and Regulate the Transformation of Free Radicals摘要:
Abstract A major issue with Fenton‐like reaction is the excessive consumption of H2O2 caused by the sluggish regeneration rate of low‐valent metal, and how to improve the activation efficiency of H2O2 has become a key in current research. Herein, a nano‐heterostructure catalyst (1.0‐MnCu/C) based on nano‐interface engineering is constructed by supporting Cu and MnO on carbon skeleton, and its kinetic rate for the degradation of tetracycline hydrochloride is 0.0436 min−1, which is 2.9 times higher than that of Cu/C system (0.0151 min−1). The enhancement of removal rate results from the introduced Mn species can aggregate and transfer electrons to Cu sites through the electron bridge Mn−N/O−Cu, thus preventing Cu2+ from oxidizing H2O2 to form O2•−, and facilitating the reduction of Cu2+ and generating more reactive oxygen species (1O2 and ·OH) with stronger oxidation ability, resulting in H2O2 utilization efficiency is 1.9 times as much as that of Cu/C. Additionally, the good and stable practical application capacity in different bodies demonstrates that it has great potential for practical environmental remediation.
DOI:10.1002/smll.202311984
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
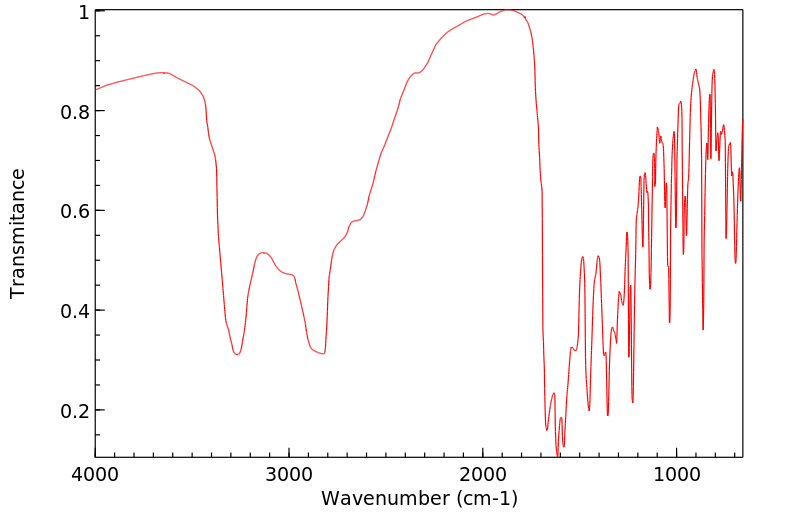
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息