2-methyl-2-propyl-oxirane | 96481-54-8
中文名称
——
中文别名
——
英文名称
2-methyl-2-propyl-oxirane
英文别名
2-propyl-2-methyloxirane;Oxirane, 2-methyl-2-propyl-;2-methyl-2-propyloxirane
CAS
96481-54-8
化学式
C6H12O
mdl
——
分子量
100.161
InChiKey
FFXMEOKFDOVSGT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:7
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12.5
-
氢给体数:0
-
氢受体数:1
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:三乙基铝与环氧化物的反应摘要:三乙基铝和1,1-二烷基环氧乙烷的主要产物(水解后)为取代的新戊醇。三乙基铝和环氧丙烷反应(摩尔比> 1)以高产率(约98%)形成2-甲基丁醇。该反应的产物取决于反应物的摩尔比。当三乙基铝/环氧丙烷的比率为0.7时,主要是2-戊醇。与顺式-和反式-2,3-环氧丁烷的反应产物(三乙基铝/环氧的摩尔比> 1)是苏基-3-甲基-2-戊醇和赤型-3-甲基-2-戊醇分别表明在接受乙基的碳上已经发生了转化。讨论了这些反应的机理。DOI:10.1016/s0022-328x(00)87832-8
-
作为产物:参考文献:名称:壬基多金属氧酸盐[γ-SiW10O34H2O2] 4-催化过氧化氢烯烃环氧化。摘要:双空Keggin型多金属氧酸盐[TBA](4)[γ-SiW(10)O(34)(H(2)O)(2)](I)的四正丁基铵(TBA)催化烯烃,烯丙醇和硫化物与30%过氧化氢水溶液的氧转移反应。对位取代的苯乙烯竞争性氧化的负Hammett rho(+)(-0.99),对于I催化的蒽5的氧化,(亲核氧化)/(总氧化)的低值(亲核氧化)/(总氧化)X.SO = 0.04。氧化物(SSO)揭示在I上形成强亲电子氧化剂。3-甲基-1-环己烯环氧化过程中反式环氧化物的优先形成证明了I活性位的空间约束。I催化的环氧化继续进行诱导期,该诱导期在用过氧化氢处理I时消失。(29)Si和(183)W NMR光谱法和CSI质谱法显示,I与过量的过氧化氢反应导致快速形成二过氧化氢物种,[TBA](4)[γ-SiW(10)O(32) (O(2))(2)](II),保留了γ-Keggin型结构。尽管分离的化合物Ⅱ对于环辛烯的DOI:10.1002/chem.200600384
文献信息
-
Catalytic multicomponent reaction between nitroalkanes, elemental sulfur, and oxiranes作者:Mehdi Khalaj、Mahboubeh Taherkhani、Fereshteh Naderi、Seyed Mahmoud Mousavi-SafaviDOI:10.1007/s00706-017-2067-9日期:2018.1nitroalkanes and elemental sulfur with oxiranes was developed with the aid of silver salt. This reaction procedure provides a novel and practical strategy for the rapid assembly of 1,3-oxathiolane skeletons. The reaction exhibited remarkable functional group tolerability and regio-selectivity so that only one regio-isomer formed during the ring opening of oxiranes. Graphical abstract
-
An efficient procedure for the synthesis of functionalized 3,4-dihydro-2H-pyrans via catalytic multicomponent reaction作者:Alireza Samzadeh-Kermani、Sajjad AtaeifarDOI:10.1007/s00706-017-2065-y日期:2018.1with reactive nitrilium ion derived from the reaction of isocyanides and oxiranes in the presence of lithium salts in PEG-400. The reaction was successfully utilized to synthesize 3,4-dihydro-2H-pyrans in accordance with a simple and environmentally benign procedure. The optimized reaction conditions allowed the selective synthesis of the highly functionalized 3,4-dihydro-2H-pyrans from the commercially
-
Copper Salt Catalyzed Synthesis of Functionalized 2 <i>H</i> ‐Pyranes作者:Alireza Varmazyar、Sajjad Sedaghat、Ahmad Nozad Goli‐Kand、Mehdi Khalaj、Samira Arab‐SalmanabadiDOI:10.1002/jhet.3570日期:2019.6oxiranes, and malonitrile has been described. In this transformation, copper acetylide was attacked on oxiranes to form homopropargyl alkoxy‐copper intermediate that was further transferred to 2H‐pyrane skeletons by reaction with malonitrile. We found that the reaction was not productive without hexafluoroisopropanol.
-
[EN] SYSTEMS AND METHODS FOR REGIOSELECTIVE CARBONYLATION OF 2,2-DISUBSTITUTED EPOXIDES<br/>[FR] SYSTÈMES ET PROCÉDÉS DE CARBONYLATION RÉGIOSÉLECTIVE D'ÉPOXYDES 2,2-DISUBSTITUÉS申请人:UNIV CORNELL公开号:WO2020102816A1公开(公告)日:2020-05-22Provided are methods of carbonylating cyclic substrates to produce carbonyl ated cyclic products. The cyclic substrates may be 2, 2-di substituted epoxides and the cyclic products may be β,β-di substituted lactones. The method may be carried out by forming and pressurizing a reaction mixture of the cyclic substrate, a solvent, carbon monoxide, and a [LA+][CO(CO)4-] catalyst, where [LA+] is a Lewis acid capable of coordinating to the cyclic substrate. The method may proceed with a regioselectivity of 90:10 or greater. The resulting carbonylated cyclic products may be converted to ketone aldol products that retain the stereochemistry and enantiomeric ratio of the carbonyl ated cyclic products.
-
Isocyanide-based multicomponent reaction for the formation of 1,3-oxathiolane-2-imine derivatives作者:Alireza Samzadeh-Kermani、Samira ZamenrazDOI:10.1007/s00706-017-1939-3日期:2017.10AbstractThe adduct of isocyanide and elemental sulfur has been employed as the isothiocyanate source in reaction with oxirane to form 1,3-oxathiolane-2-imine derivatives. The optimum conditions are developed using HMPA at 55 °C for 12 h. Various isocyanides and oxiranes were tolerated under the optimum conditions. Graphical abstract
表征谱图
-
氢谱1HNMR
-
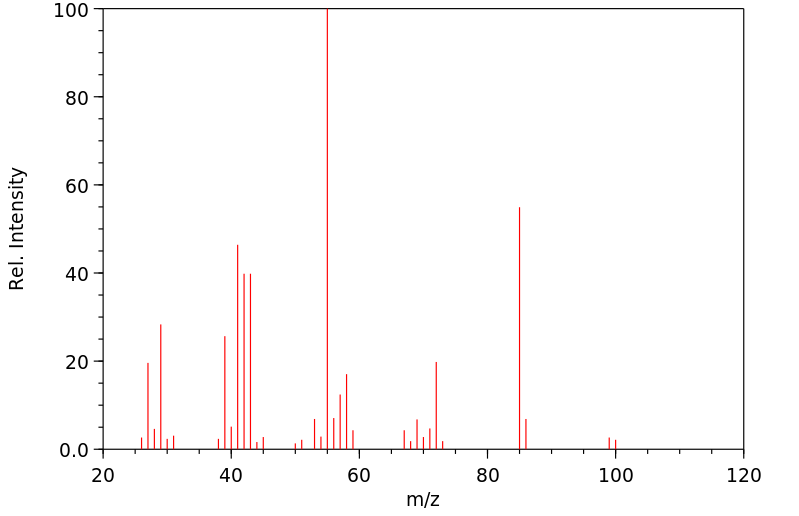
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷