4-methyl-1-phenyl-[1,2,4]triazolo[4,3-a]quinoxaline | 19848-92-1
中文名称
——
中文别名
——
英文名称
4-methyl-1-phenyl-[1,2,4]triazolo[4,3-a]quinoxaline
英文别名
4-Methyl-1-phenyl-s-triazolo<4,3-a>chinoxalin;1-Phenyl-4-methyl-1,2,4-triazolo<4,3-a>chinoxalin;1-phenyl-4-methyl-1,2,4-triazolo[4,3-a]quinoxaline;4-Methyl-1-phenyl(1,2,4)triazolo(4,3-a)quinoxaline
CAS
19848-92-1
化学式
C16H12N4
mdl
——
分子量
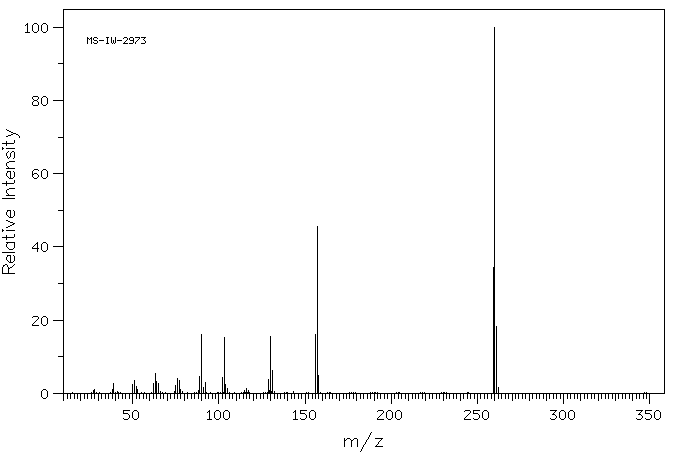
260.298
InChiKey
WBEHLJGGQKPBGY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:205-206 °C
-
密度:1.30±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:20
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:43.1
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为产物:描述:2-氯-3-甲基喹喔啉 在 一水合肼 作用下, 以 乙醇 、 乙腈 为溶剂, 反应 2.0h, 生成 4-methyl-1-phenyl-[1,2,4]triazolo[4,3-a]quinoxaline参考文献:名称:[EN] TRIAZOLOPYRAZINE DERIVATIVES AND THEIR USE FOR TREATING NEUROLOGICAL AND PSYCHIATRIC DISORDERS
[FR] DÉRIVÉS DE TRIAZOLOPYRAZINE ET LEUR UTILISATION POUR LE TRAITEMENT DE TROUBLES NEUROLOGIQUES ET PSYCHIATRIQUES摘要:本发明涉及式(I)的三唑吡嗪化合物。该发明的不同方面涉及包含所述化合物的药物组合物以及将该化合物用作治疗神经系统和精神疾病的治疗剂的用途。公开号:WO2013034755A1
文献信息
-
Hypervalent Iodine‐Mediated Synthesis of 1‐Aryl‐4‐methyl‐1,2,4‐triazolo[4,3‐a]quinoxalines by Oxidative Cyclization of Arene Carbaldehyde‐3‐methylquinoxalin‐2‐yl Hydrazones作者:Ranjana Aggarwal、Garima SumranDOI:10.1080/00397910600602586日期:2006.7Abstract Arene carbaldehyde‐3‐methylquinoxalin‐2‐yl hydrazones (2) obtained by the condensation of 2‐hydrazino‐3‐methylquinoxaline (1) with various aromatic aldehydes, on treatment with iodobenzene diacetate (IBD) in dichloromethane, undergo oxidative cyclization to exclusively afford 1‐aryl‐4‐methyl‐1,2,4‐triazolo[4,3‐a]quinoxalines (5) in excellent yield.
-
Synthesis of Triazoloquinoxalines as Antitubercular Agents作者:Kondapalli Venkata Gowri Chandra Sekhar、Vajja Sambasiva Rao、Dalip KumarDOI:10.5012/bkcs.2011.32.8.2657日期:2011.8.201,2,4-Triazoles and quinoxalines were found to display various pharmacological activities. Hence a series of 1-aryl-4-methyl-1,2,4-triazolo[4,3-a]quinoxalines were synthesized. Due to various advantages of organic reactions under solvent-free conditions these compounds were developed using iodobenzene diacetate under solvent-free conditions. The synthesized compounds were characterized by elemental microanalysis, infrared spectroscopy,
$^1H$ NMR,$^13}C$ NMR and HRMS. All the synthesized compounds were investigated for their antitubercular activity and 5g was found to the most active compound. -
Some novel observations on the reaction of 2-hydrazino-3-methylquinoxaline with trifluoromethyl-β-diketones作者:Ranjana Aggarwal、Rajiv Kumar、Shiv P. SinghDOI:10.1016/j.jfluchem.2009.06.021日期:2009.10The reaction of 2-hydrazino-3-methylquinoxaline 1 with trifluoromethyl-beta-diketones 2 not only yields the expected 5-trifluoromethyl-5-hydroxy-Delta(2)-pyrazolines 3a-3f and/or 3-trifluoromethylpyrazoles 4c-4f but also the unexpected products 1,2,4-triazolo[4,3-a]quinoxalines 5a-5f and/or 3(5)-trifluoromethyl-1H-pyrazoles 6c-6f. Furthermore, the acid-catalyzed dehydration of 5-hydroxypyrazolines 3a-3b resulted in the formation of unexpected 5a-5b along with the expected corresponding pyrazoles 7a-7b. These unprecedented observations provide evidence for the existence of equilibrium between the hydroxypyrazoline 3 and its open chain tautomer, ketoimine 9 in the mechanistic path leading to the formation of pyrazoles 7 and triazoles 5. (C) 2009 Elsevier B.V. All rights reserved.
-
KOENNECKE A.; LIPPMANN E., Z. CHEM., 1978, 18, NO 5, 175-177作者:KOENNECKE A.、 LIPPMANN E.DOI:——日期:——
-
1-ARYL-4-METHYL-[1,2,4]TRIAZOLO[4,3-a]QUINOXALINE DERIVATIVES申请人:Andrés-Gil José Ignacio公开号:US20140147386A1公开(公告)日:2014-05-29The present invention relates to novel 1-aryl-4-methyl-[1,2,4]triazolo[4,3-a]-quinoxaline derivatives as inhibitors of phosphodiesterase 2 (PDE2) and to a lesser extent of phosphodiesterase 10 (PDE10) or as inhibitors of both, phosphodiesterases 2 and 10. The invention is also directed to pharmaceutical compositions comprising such compounds, to processes for preparing such compounds and compositions, and to the use of such compounds and compositions for the prevention and treatment of disorders in which PDE2 is involved, or disorders in which both PDE2 and PDE 10 are involved, such as neurological and psychiatric disorders, and endocrinological or metabolic diseases. The present invention also relates to radiolabelled compounds which may be useful for imaging and quantifying the PDE2 enzyme in tissues, using positron-emission tomography (PET). The invention is also directed to compositions comprising such compounds, to processes for preparing such compounds and compositions, to the use of such compounds and compositions for imaging a tissue, cells or a host, in vitro or in vivo and to precursors of said compounds.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
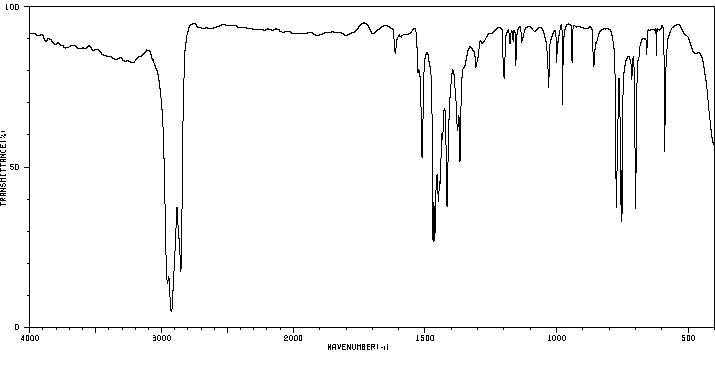
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)