1,3-diphenyldisiloxane | 17962-59-3
中文名称
——
中文别名
——
英文名称
1,3-diphenyldisiloxane
英文别名
DPDS;Phenyl(phenylsilyloxy)silane
CAS
17962-59-3
化学式
C12H14OSi2
mdl
——
分子量
230.414
InChiKey
HJJVSRYKOMHEGK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:86-90 °C(Press: 0.15 Torr)
计算性质
-
辛醇/水分配系数(LogP):-0.18
-
重原子数:15
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,3-二苯基-1,3-二甲基二硅烷 1,3-dimethyl-1,3-diphenyldisiloxane 6689-22-1 C14H18OSi2 258.467
反应信息
-
作为反应物:参考文献:名称:The Cleavage of sym-Diphenyldisiloxane by Organometallic Compounds摘要:DOI:10.1021/ja01563a043
-
作为产物:描述:参考文献:名称:从硅烷中氢化二硅氧烷的可扩展合成:从苯基硅烷中单锅法制备 1,3-二苯基二硅氧烷摘要:抽象 提出了一种简单的、一锅法和高产率的 1,3-二苯基二硅氧烷合成法。类似的对称二硅氧烷材料的制备也是用相同的方案完成的。这种机械化学程序高效且高度可扩展,为从广泛可获得的市售硅烷中获得氢基二氧烷提供了一条便捷的途径。 提出了一种简单的、一锅法和高产率的 1,3-二苯基二硅氧烷合成法。类似的对称二硅氧烷材料的制备也是用相同的方案完成的。这种机械化学程序高效且高度可扩展,为从广泛可获得的市售硅烷中获得氢基二氧烷提供了一条便捷的途径。DOI:10.1055/s-0036-1588580
-
作为试剂:描述:参考文献:名称:1,3-二苯基二硅氧烷化学选择性还原氧化膦摘要:将氧化膦还原为相应的膦是制备这些有价值的试剂的最直接的方法。然而,由于P=O键的强度和/或较差的原子经济性,现有的还原氧化膦的方法存在化学选择性不足的问题。在此,我们报告了迄今为止用于这种转化的最强大的化学选择性还原剂的发现,1,3-二苯基二硅氧烷(DPDS)。无添加剂的DPDS选择性还原仲膦氧化物和叔膦氧化物,即使存在醛、硝基、酯、α,β-不饱和羰基、偶氮羧酸酯和氰基官能团,也能保持构型。阿累尼乌斯分析表明, DPDS还原的活化势垒明显低于任何先前计算的硅烷还原系统。催化布朗斯台德酸的加入进一步降低了活化能垒,并首次在室温下实现了硅烷介导的无环氧化膦还原。DOI:10.1002/chem.201703875
文献信息
-
The Trityl‐Cation Mediated Phosphine Oxides Reduction作者:Claire Laye、Jonathan Lusseau、Frédéric Robert、Yannick LandaisDOI:10.1002/adsc.202100189日期:2021.6.21Reduction of phosphine oxides into the corresponding phosphines using PhSiH3 as a reducing agent and Ph3C+[B(C6F5)4]− as an initiator is described. The process is highly efficient, reducing a broad range of secondary and tertiary alkyl and arylphosphines, bearing various functional groups in generally good yields. The reaction is believed to proceed through the generation of a silyl cation, which reaction
-
A more critical role for silicon in the catalytic Staudinger amidation: silanes as non-innocent reductants作者:Keith G. Andrews、Ross M. DentonDOI:10.1039/c7cc03076b日期:——
We demonstrate that
in situ -generated silyl ester intermediates are key mediators of the catalytic, traceless Staudinger amidation reaction.我们展示了原位生成的硅酯中间体是催化的、无痕迹的施陀林酰胺化反应的关键中介体。 -
Designing a Cr-catalyst bearing redox non-innocent phenalenyl-based ligand towards hydrosilylative CO<sub>2</sub> functionalization
-
Chemoselective Reduction of Tertiary Amides by 1,3-Diphenyldisiloxane (DPDS)作者:Travis A. Hammerstad、Pooja V. Hegde、Courtney C. Aldrich、Kathleen J. WangDOI:10.1055/a-1709-3426日期:2022.5A convenient procedure for the chemoselective reduction of tertiary amides at room temperature in the presence of air and moisture using 1,3-diphenyldisiloxane (DPDS) is developed. The reaction conditions tolerate a significant number of functional groups including esters, nitriles, secondary amides, carbamates, sulfoxides, sulfones, sulfonyl fluorides, halogens, aryl-nitro groups, and arylamines.
-
Metal‐Ligand Cooperativity in Mn <sup>I</sup> ‐Catalysed N‐Formylation of Secondary Amides and Lactams Using CO <sub>2</sub> at Room Temperature作者:Soumi Chakraborty、Rounak Nath、Anuj Kumar Ray、Ankan Paul、Swadhin K. MandalDOI:10.1002/chem.202202710日期:2023.2A MnI complex coordinated with a redox non-innocent phenalenyl (PLY) ligand is reported for catalytic N-formylation of secondary amides (including late-stage diversification of pharmaceutics) and lactams with CO2 as C1 source at room temperature for the first time. A combined approach of experimental analysis and DFT calculations sketch the plausible radical-mediated pathway of an unconventional metal-ligand
表征谱图
-
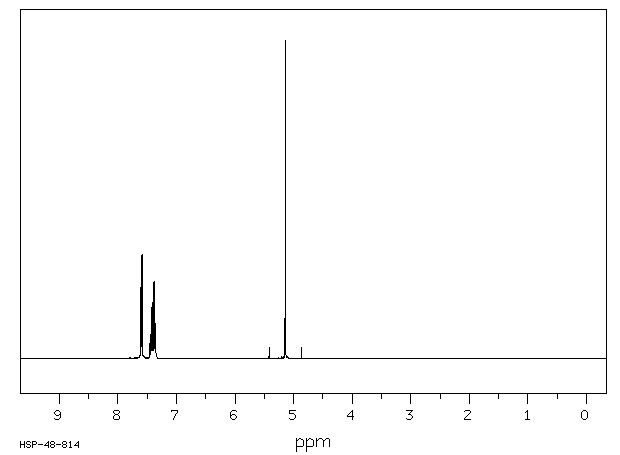
氢谱1HNMR
-
质谱MS
-
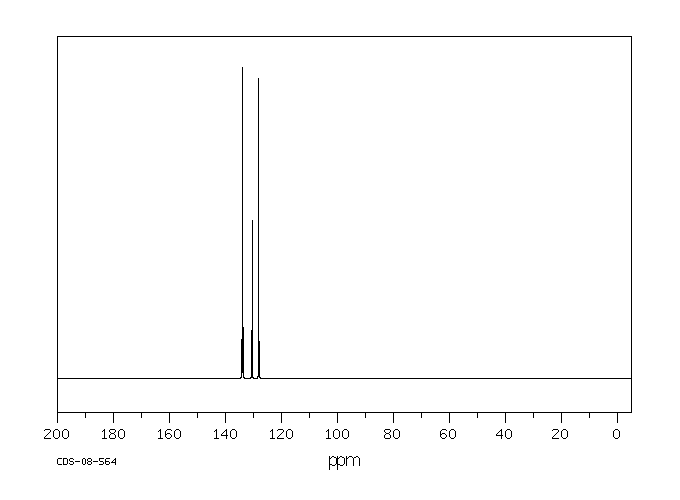
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫