diphenylcarbamoyl azide | 17223-83-5
中文名称
——
中文别名
——
英文名称
diphenylcarbamoyl azide
英文别名
Diphenyl-carbamoylazid;(3E)-3-diazo-1,1-diphenylurea
CAS
17223-83-5
化学式
C13H10N4O
mdl
——
分子量
238.249
InChiKey
FTGQOWKFJNMITD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:18
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.7
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,1-二苯基脲 N,N-diphenylurea 603-54-3 C13H12N2O 212.251 4,4-二苯氨基脲 N,N-diphenyl semicarbazide 603-51-0 C13H13N3O 227.266 二苯氨基甲酰氯 diphenylcarbamic chloride 83-01-2 C13H10ClNO 231.681 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三苯基脲 N,N,N'-triphenylurea 5663-04-7 C19H16N2O 288.349 —— 3-allyl-1,1-diphenylurea 101279-92-9 C16H16N2O 252.316 —— N'-pentyl-N,N-diphenyl-urea 101785-10-8 C18H22N2O 282.385 —— N'-benzyl-N,N-diphenyl-urea 102002-70-0 C20H18N2O 302.376 —— N,N-diphenylcarbamoylpyrrolidine 6123-91-7 C17H18N2O 266.343 N,N-二苯基吗啉-4-甲酰胺 N,N-diphenylmorpholine-4-carboxamide 75125-45-0 C17H18N2O2 282.342 N,N-二苯基哌啶-1-甲酰胺 piperidine-1-carboxylic acid diphenylamide 75534-73-5 C18H20N2O 280.37
反应信息
-
作为反应物:描述:参考文献:名称:Stolle, Chemische Berichte, 1924, vol. 57, p. 1065摘要:DOI:
-
作为产物:描述:参考文献:名称:Art As Religious Commitment: Kafka’s Debt to Kierkegaardian Ideas and their Impact on his Late Stories摘要:Although Kafka’s reception of Kierkegaardian ideas has received much critical attention the critics have so far paid little heed to similarities between Kierke‐gaard’s religious and Kafka’s aesthetic views. My intention in the following is to show that in spite of Kafka’s critical remarks on his philosophy, Kierkegaard’s definition of a religious person influenced his description of the artist’s existence in Erstes Leid (1922), Ein Hungerkünstler (1922) and Josefine, die Sängerin oder das Volk der Mäuse (1924). In these stories Kafka turns Kierkegaard’s ideas about spiritual inwardness and passionate attitude towards religious life into artistic inwardness and passionate attitude towards art. He also describes how devotion that these artists feel towards their art leads to their solitude and how their lives reflect suffering, doubt and despair which is similar to Kierkegaard’s description of religious suffering. Kafka’s critical remarks on Kierkegaard’s philosophy should therefore be understood as a clear rejection of Kierkegaard’s Protestant theology, although these same ideas gave him inspiration to formulate his views on the artist’s existence.DOI:10.1111/1468-0483.00182
文献信息
-
A Synthetic Approach to <i>N</i>-Aryl Carbamates via Copper-Catalyzed Chan–Lam Coupling at Room Temperature作者:Soo-Yeon Moon、U. Bin Kim、Dan-Bi Sung、Won-Suk KimDOI:10.1021/jo502828r日期:2015.2.6catalyst. The reaction proceeds readily in an open flask at room temperature without additional base, ligand, or additive. Rapid access to urea analogues via a two-step one-pot procedure is enabled by reacting N-arylcarbamates with aluminum–amine complexes. In addition, among several boronic acid derivatives prepared, dimethylphenyl boronate was found to react rapidly in its reaction with benzyl azidoformate
-
Ir-Catalyzed C–H Amidation Using Carbamoyl Azides for the Syntheses of Unsymmetrical Ureas作者:Kwangho Yoo、Jooyeon Lee、Myung Hwan Park、Youngjo Kim、Hyun Jin Kim、Min KimDOI:10.1021/acs.joc.0c00659日期:2020.5.1An iridium-catalyzed C-H amidation for the syntheses of unsymmetrical urea was developed using carbamoyl azides (R(R')N-C(O)-N3) as the nitrogen source. A combination of iridium and silver gave an active catalyst for C-N bond formation. A variety of urea derivatives were synthesized using carbamoyl azides with only dinitrogen byproducts. Finally, the use of the transient directing group strategy with
-
1,2,3-Triazoles from carbonyl azides and alkynes: filling the gap作者:Estela Haldón、Eleuterio Álvarez、M. Carmen Nicasio、Pedro J. PérezDOI:10.1039/c4cc03614j日期:——Electron deficient azides are challenging substrates in CuAAC reactions. Particularly, when N-carbonyl azides are applied the formation of N-carbonyl triazoles has not yet been observed. We report herein the first example of this class of reaction, with a copper-based system that efficiently enables the synthesis of N-carbamoyl 1,2,3-triazoles by [3+2] cycloaddition of N-carbamoyl azides and alkynes.
-
[EN] ACTIVATORS OF SOLUBLE GUANYLATE CYCLASE<br/>[FR] ACTIVATEURS DE GUANYLATE CYCLASE SOLUBLE申请人:UNIV LONDON公开号:WO2000027394A1公开(公告)日:2000-05-18The present invention describes the use of pyrazole or indazole derivates as activators of soluble guanylate cyclase. Some compounds disclosed are novel. All activators are vasodilators and/or inhibit platelet aggregation and are therefore useful in the treatment of peripheral vascular diseases such as hypertension, angina pectoris or atherosclerosis, or in the treatment of prevention of glaucoma, preeclampsia, Raynaud's syndrome, stroke or erectile disfunctions.
-
Synthesis and Biological Evaluation of Novel Pyrazoles and Indazoles as Activators of the Nitric Oxide Receptor, Soluble Guanylate Cyclase作者:David L. Selwood、David G. Brummell、Joanna Budworth、Guillaume E. Burtin、Richard O. Campbell、Surinder S. Chana、Ian G. Charles、Patricia A. Fernandez、Robert C. Glen、Maria C. Goggin、Adrian J. Hobbs、Marcel R. Kling、Qian Liu、David J. Madge、Sylvie Meillerais、Kenneth L. Powell、Karen Reynolds、Graham D. Spacey、Jeremy N. Stables、Mark A. Tatlock、Kerry A. Wheeler、Grant Wishart、Chi-Kit WooDOI:10.1021/jm001034k日期:2001.1.1Database searching and compound screening identified 1-benzyl-3-(3-dimethylaminopropyloxy)-indazole (benzydamine, 3) as a potent activator of the nitric oxide receptor, soluble guanylate cyclase. A comprehensive structure-activity relationship study surrounding 3 clearly showed that the indazole C-3 dimethylaminopropyloxy substituent was critical for enzyme activity. However replacement of the indazole ring of 3 by appropriately substituted pyrazoles maintained enzyme activity. Compounds were evaluated for inhibition of platelet aggregation and showed a general lipophilicity requirement. Aryl-substituted pyrazoles 32, 34, and 43 demonstrated potent activation of soluble guanylate cyclase and potent inhibition of platelet aggregation. Pharmacokinetic studies in rats showed that compound 32 exhibits modest oral bioavailability (12%). Furthermore 32 has an excellent selectivity profile notably showing no significant inhibition of phosphodiesterases or nitric oxide synthases.
表征谱图
-
氢谱1HNMR
-
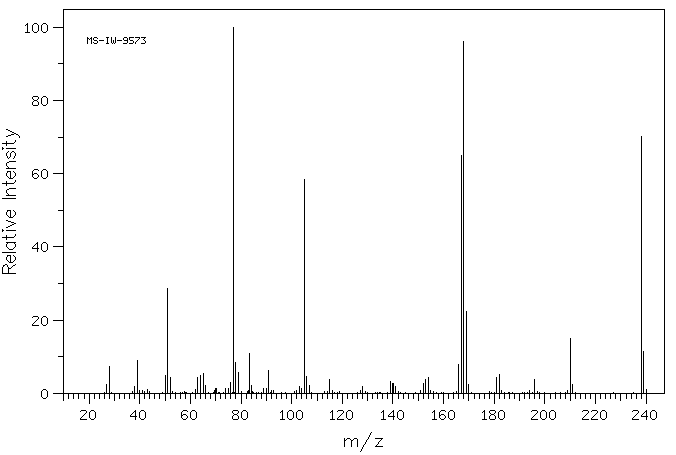
质谱MS
-
碳谱13CNMR
-
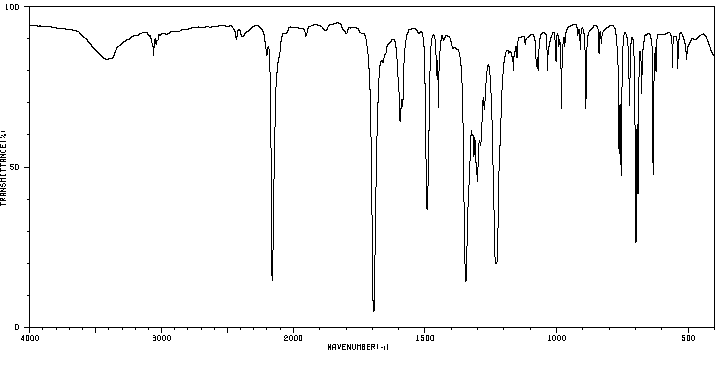
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫