diethyl-2,3-dibenzoylsuccinate | 4687-38-1
分子结构分类
中文名称
——
中文别名
——
英文名称
diethyl-2,3-dibenzoylsuccinate
英文别名
diethyl α,α'-dibenzoylsuccinate;diethyl 2,3-dibenzoylsuccinate;diethyl 2,3-benzoylsuccinate;2,3-dibenzoyl-succinic acid diethyl ester;2,3-Dibenzoyl-bernsteinsaeure-diaethylester;meso-2,3-Dibenzoyl-bernsteinsaeure-diaethylester;diethyl 2,3-dibenzoylbutanedioate
CAS
4687-38-1
化学式
C22H22O6
mdl
——
分子量
382.413
InChiKey
LNKDGSQQKJLYEV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:28
-
可旋转键数:11
-
环数:2.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:86.7
-
氢给体数:0
-
氢受体数:6
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Rubin, Mordecai B.; Bargurie, Moshe; Kosti, Sonia, Journal of the Chemical Society. Perkin transactions I, 1980, p. 2670 - 2677摘要:DOI:
-
作为产物:参考文献:名称:硝酸铈 (IV) 铵氧化偶联 1,3-二羰基化合物摘要:描述了一种使用硝酸铈铵在 CH3CN/CH3OH/H2O 中进行 1,3-二羰基化合物分子间偶联的快速、温和且高效的方法。该过程为合成 1,4-二酮衍生物提供了一种简便的方法,收率高达 96%,并为硝酸铈铵介导的氧化机制提供了有力证据。DOI:10.1080/00397910903161876
文献信息
-
一种可见光催化的1,3-二羰基类化合物脱氢 自偶联的方法
-
Ionic Liquid as Catalyst and Reaction Medium: A Simple and Efficient Procedure for Paal–Knorr Furan Synthesis作者:Gangqiang Wang、Zhi Guan、Rongchang Tang、Yanhong HeDOI:10.1080/00397910902978049日期:2010.1.14The ionic liquid 1-butyl-3-methyl-imidazolium hydrogen sulfate, [bmim]HSO4, efficiently catalyzes Paal–Knorr furan synthesis without any organic solvent. A wide range of aliphatic and aromatic 1,4-diketones easily undergo condensations to form furan derivatives, providing a general and convenient procedure. The Paal–Knorr reaction of ester-substituted 1,4-diketones is first reported. The ionic liquid
-
An Efficient Procedure for the Synthesis of Polysubstituted Pyrroles in an Ionic Liquid作者:Yan-Hong He、Gang-Qiang Wang、Ke-Ling Xu、Zhi GuanDOI:10.1515/znb-2011-0212日期:2011.2.11-butyl-3-methyl-imidazolium hydrogen sulfate, [bmim]HSO4, was used as a catalyst and reaction medium for the pyrrole synthesis, and a wide range of aliphatic, aromatic, heteroaromatic and carboxylic 1,4-diketones easily underwent condensations with aniline and ethylenediamine to form polysubstituted pyrroles. Sequential decarboxylation/Paal-Knorr pyrrole condensation was observed, which provides a new
-
Ambient-Light-Promoted Three-Component Annulation: Synthesis of Perfluoroalkylated Pyrimidines作者:Rui Wang、Wei Guan、Zheng-Bo Han、Fushun Liang、Takeo Suga、Xihe Bi、Hiroyuki NishideDOI:10.1021/acs.orglett.7b00894日期:2017.5.5reaction of active methylene compounds, perfluoroalkyl iodides, and guanidines/amidines is reported. This constitutes a powerful method to prepare perfluoroalkylated pyrimidines with mild reaction conditions, broad substrate scope, excellent functional group tolerance, and simple operation. A radical/polar mechanism involving the formation of a halogen-bond adduct and radical cross-coupling is proposed
-
A Modification of the Knorr Oxidative Coupling Method for Preparation of 1,4-Diketones作者:Anxin Wu、Yurui Zhao、Ning Chen、Xinfu PanDOI:10.1080/00397919708005036日期:1997.1Abstract The oxidative coupling of β-keto esters has been achieved in good yield using NaOEt / 12.摘要 使用 NaOEt / 12 以良好的收率实现了 β-酮酯的氧化偶联。
表征谱图
-
氢谱1HNMR
-
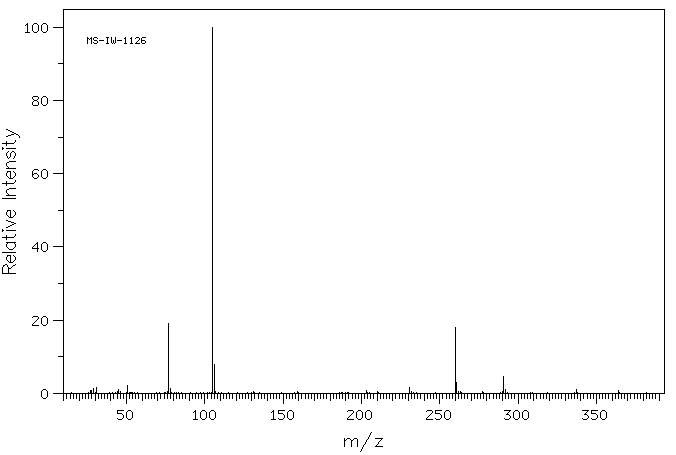
质谱MS
-
碳谱13CNMR
-
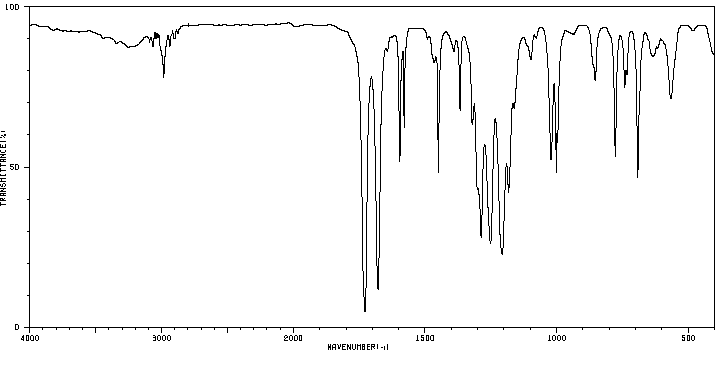
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
苯基(2,4,5-三苯基-2-环戊烯-1-基)甲酮
肠二醇
珠子草素
开环异落叶松树脂酚
安五脂素
外消旋肠二醇-13C3
去甲二氢愈创木酸
半去甲二氢愈创木酸
二甲基2,5-二苯基-3,4-二氢-2H-吡咯-3,4-二羧酸酯
二氢荜澄茄脂素
9,9'-二-O-(E)-阿魏酰开环异落叶松脂素
5,6-二甲基-5-苯基-1,3-环己二烯
4-[4-(4-羟基-3-甲氧基苯基)-2,3-二甲基丁基]-2-甲氧基苯酚
2-甲氧基-1,2,3,4-四苯基丁烷-1,4-二酮
2,6-二甲氧基-4-羟基苯甲醛
2,6-二甲氧基-4-[(2R,3R)-4-甲氧基-3-[(7-甲氧基-1,3-苯并二噁唑-5-基)甲基]-2-(甲氧基甲基)丁基]苯酚
2,3-双[(4-羟基-3-甲氧基苯基)甲基]丁烷-1,4-二醇
2,3-双[(3,4-二甲氧基苯基)甲基]丁烷-1,4-二醇
2,3-双[(3,4-二甲氧基苯基)甲基]丁二酸
2,3-双(4-羟基-3-甲氧基苄基)琥珀酸二甲酯
2,3-双(3,4-二甲氧基苄基)-4-甲氧基-4-氧代丁酸
2,3-二苄基丁烷-1,4-二醇
2,3-二甲基-1,4-二苯基丁烷-2,3-二醇
2,3-二甲基-1,4-二苯基丁烷-1,4-二酮
2,3-二异丙基-1,2-二苯基-1,4-丁二酮
2,3-二[(3-羟基苯基)甲基]丁烷-1,4-二醇
2,2,3,3-四甲基-1,4-二苯基丁烷-1,4-二酮
1,4-丁烷二酮
1,2,3,4-四苯基环戊烷
1,2,3,4-四苯基丁烷
1,2,3,4-四苯基-1,4-丁烷二酮
1,2,3,4-四-(4-甲氧基-苯基)-丁烷-1,4-二酮
1,1'-[(2S,3S)-2,3-双(甲氧基甲基)-1,4-丁二基]双[3,4-二甲氧基苯]
1,1'-(2,3-二甲基-1,4-丁烷二基)二(3,4-二甲氧基苯)
1,1',2,2',3,3'-六苯基-1,1'-联(2-环丙烯)
(3,3,4,4-四甲基-2-苯基环丁烯-1-基)苯
(2R,3S)-1,4-二(4-羟基-3-甲氧基苯基)-2,3-二甲基丁烷-1-酮
(-)-二氢愈创木脂酸
(+)-开环异落叶松树脂酚
(2S,3R)-2,3-Bis-(3,5-di-tert-butyl-4-hydroxy-benzyl)-butane-1,4-diol
(3-{1-[(E)-2-(4-Methoxy-phenyl)-1-methyl-vinyl]-3,3-dimethyl-1,3-dihydro-isobenzofuran-1-yl}-propyl)-dimethyl-amine
(6-Benzoyl-1,4-diphenyl-3,6-di-p-tolyl-tetrahydro-isoxazolo[4,3-c]isoxazol-3-yl)-phenyl-methanone
2-(1,3-Diphenylpropan-2-yl)-2-methylpropane-1,3-diol
1-hydroxy-6-(3-oxo-3-phenylpropyl)-4,4-diphenyl-2,3-dioxabicyclo[4.3.0]nonan-7-one
2-[2-(3,4-dimethoxy-phenyl)-1-methyl-ethyl]-3-phenyl-oxaziridine
1,3,5-triphenyl-pyrrolidine-2,2,4-tricarboxylic acid trimethyl ester
3-Methyl-1-phenyl-cyclopropane-1,2-dicarboxylic acid diethyl ester
2-Benzoyl-3-(4-methoxy-phenyl)-5-oxo-5-p-tolyl-pentanoic acid ethyl ester