5-乙酯基-2-硫代尿嘧啶 | 38026-46-9
中文名称
5-乙酯基-2-硫代尿嘧啶
中文别名
2-硫代尿嘧啶-5-甲酸乙酯
英文名称
ethyl 2-thiouracil-5-carboxylate
英文别名
ethyl 4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate;5-carbethoxy-2-thiouracil;5-carboethoxy-2-thiouracil;ethyl 4-oxo-2-sulfanylidene-1H-pyrimidine-5-carboxylate
CAS
38026-46-9
化学式
C7H8N2O3S
mdl
MFCD09743156
分子量
200.218
InChiKey
FQFSHLBWRUOCPX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:249-255 °C (dec.)(lit.)
-
密度:1.4342 (rough estimate)
-
溶解度:0.01 M
-
稳定性/保质期:
常规情况下不会分解,也没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:99.5
-
氢给体数:2
-
氢受体数:4
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
RTECS号:UV7775000
-
海关编码:2933599090
-
储存条件:密封、阴凉、干燥处保存。
SDS
| Name: | 5-Carbethoxy-2-Thiouracil 99% Material Safety Data Sheet |
| Synonym: | Ethyl 2-Thiouracil-5-Carboxylate |
| CAS: | 38026-46-9 |
Synonym:Ethyl 2-Thiouracil-5-Carboxylate
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 38026-46-9 | 5-Carbethoxy-2-Thiouracil | 99% | 253-749-2 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 38026-46-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: light orange
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 249.00 - 255.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H8N2O3S
Molecular Weight: 200.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 38026-46-9: UV7775000 LD50/LC50:
Not available.
Carcinogenicity:
5-Carbethoxy-2-Thiouracil - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 38026-46-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 38026-46-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 38026-46-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,4-二氢-2-甲巯基-4-氧代-5-嘧啶甲酸乙酯 ethyl 4-hydroxy-2-(methylthio)pyrimidine-5-carboxylate 53554-29-3 C8H10N2O3S 214.245 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-硫脲嘧啶-5-羧酸 5-carboxy-2-thiouracil 23945-50-8 C5H4N2O3S 172.164 4-羟基-5-嘧啶甲酸乙酯 ethyl 3,4-dihydro-4-oxopyrimidine-5-carboxylate 4786-52-1 C7H8N2O3 168.152 5-尿嘧啶甲酸乙酯 5-carbethoxyuracil 28485-17-8 C7H8N2O4 184.152 1,4-二氢-2-甲巯基-4-氧代-5-嘧啶甲酸乙酯 ethyl 4-hydroxy-2-(methylthio)pyrimidine-5-carboxylate 53554-29-3 C8H10N2O3S 214.245 5-羟基甲基-2-硫尿嘧啶 4-Hydroxy-5-(hydroxymethyl)-2-mercaptopyrimidine 93185-31-0 C5H6N2O2S 158.181 6-氧代-1,6-二氢嘧啶-5-甲酸 6-oxo-1,6-dihydropyrimidine-5-carboxylic acid 65754-04-3 C5H4N2O3 140.098 —— ethyl 2-benzylthio-6-oxo-1,6-dihydropyrimidine-5-carboxylate 93185-32-1 C14H14N2O3S 290.343 4-羟基-2-苯基嘧啶-5-甲酸乙酯 ethyl 6-oxo-2-phenyl-1,6-dihydropyrimidine-5-carboxylate 55613-22-4 C13H12N2O3 244.25 4-羟基-2-(甲硫基)嘧啶-5-羧酸 4-hydroxy-2-(methylthio)pyrimidine-5-carboxylic acid 397308-78-0 C6H6N2O3S 186.191
反应信息
-
作为反应物:描述:参考文献:名称:Studies on fluorinated pyrimidines. II. Synthesis and antitumor activity of 5-fluoro-6-substituted-5,6-dihydrouracil-5-carboxylic acid derivatives.摘要:一类潜在的抗肿瘤药物5-氟尿嘧啶(5-FU)和1-(2-四氢呋喃基)-5-氟尿嘧啶(氟尿嘧啶)的前药,即在C-5位具有烷氧羰基、取代的氨基甲酰基或氰基,并在C-6位具有各种取代基(如烷氧基、取代巯基、取代氨基、酰氨基、亚烷基氨基氧和亚芳基氨基氧)的5-氟-5,6-二氢尿嘧啶衍生物,已被合成。本文描述了这些化合物对小鼠白血病P388或L1210的抗肿瘤活性和对灰霉病菌(Botrytis cinerea)的抗真菌活性。DOI:10.1248/cpb.30.887
-
作为产物:参考文献:名称:用于从乙氧基甲基丙二酸二乙酯和硫脲制备 5-羧基-2-硫脲嘧啶的一步或两步酸介导的环缩缩合工艺摘要:本文描述了酸介导的 5-carbethoxy-2-thiouracils 合成,通过乙氧基亚甲基丙二酸二乙酯和硫脲的一步环缩合反应,或在较温和的反应条件下,也获得了硫脲亚甲基丙二酸酯。基于嘧啶酮的衍生物具有重要的生物学意义,因为它们表现出广泛且不断增长的抗菌、抗病毒和抗癌活性。特别是 2-硫尿嘧啶衍生物作为抗 HIV 药物引起了很多治疗兴趣。具体而言,一些 S 取代的 2-硫尿嘧啶对感染 HIV-1 类型的细胞系显示出有效的逆转录酶抑制活性和抗病毒特性。同时,几个 5,已在结构单元 SC(=N-)N 显示出杀利什曼原虫和免疫刺激特性的一般范围内评估了 6-取代的 2-硫尿嘧啶的体内杀利什曼原虫活性。乙氧基亚甲基丙二酸二乙酯 (DEMM) 经常被用于制剂中不同的杂环,但仅发表了一些关于此的评论。胺与活性炭碳双键的加成是迈克尔加成的一种变体。在通过酸性催化生成伯胺的情况下,单加成产物在没有催化DOI:10.1515/hc.2007.13.4.229
文献信息
-
[EN] MODULATORS OF HSD17B13 AND METHODS OF USE THEREOF<br/>[FR] MODULATEURS DE HSD17B13 ET LEURS PROCÉDÉS D'UTILISATION申请人:REGENERON PHARMA公开号:WO2021003295A1公开(公告)日:2021-01-07The disclosure relates to compounds and pharmaceutical compositions capable of modulating the hydroxysteroid 17-beta dehydrogenase (HSD17B) family member proteins including inhibiting the HSD17B member proteins, e.g. HSD17B13. The disclosure further relates to methods of treating liver diseases, disorders, or conditions with the compounds and pharmaceutical compositions disclosed herein, in which the HSD17B family member protein plays a role.
-
A novel class of Plasmodial ClpP protease inhibitors as potential antimalarial agents作者:Sourabh Mundra、Vandana Thakur、Angelica M. Bello、Sumit Rathore、Mohd Asad、Lianhu Wei、Jane Yang、Sai Kumar Chakka、Radhakrishnan Mahesh、Pawan Malhotra、Asif Mohmmed、Lakshmi P. KotraDOI:10.1016/j.bmc.2017.08.049日期:2017.10approaches to identify a novel pyrimidine series of compounds inhibiting P. falciparum ClpP protease activity and evaluated their antiparasitic activities. Structure-activity relationship indicated that morpholine moiety at C2, an aromatic substitution at N3 and a 4-oxo moiety on the pyrimidine are important for potent inhibition of ClpP enzyme along with antiparasiticidal activity. Compound 33 exhibited
-
[EN] PYRIMIDINONE COMPOUNDS FOR USE IN THE TREATMENT OF DISEASES OR CONDITIONS MEDIATED BY LP - PLA2<br/>[FR] COMPOSÉS DE PYRIMIDINONE UTILES DANS LE TRAITEMENT DE MALADIES OU D'ÉTATS PATHOLOGIQUES INDUITS PAR LA LP-PLA2申请人:GLAXO GROUP LTD公开号:WO2012076435A1公开(公告)日:2012-06-14The present invention relates to novel compounds that inhibit Lp-PLA2 activity, processes for their preparation, to compositions containing them and to their use in the treatment of diseases associated with the activity of Lp-PLA2, for example atherosclerosis, Alzheimer's disease, and/or diabetic macular edema (I).
-
[EN] THIAZOLO(3,2-A) PYRIMIDINONE AND OTHER HETEROBICYCLIC PYRIMIDINONE COMPOUNDS FOR USE IN MEDICAL THERAPY<br/>[FR] THIAZOLO(3,2-A)PYRIMIDINONE ET AUTRES COMPOSÉS HÉTÉROBICYCLIQUES DE PYRIMIDINONE POUR UTILISATION À DES FINS THÉRAPEUTIQUES申请人:LYSOSOMAL THERAPEUTICS INC公开号:WO2017040879A1公开(公告)日:2017-03-09The invention provides heterobicyclic pyrimidinone compounds such as thiazolo[3,2-a]pyrimidinone compounds, compositions containing such compounds, medical kits, and methods for using such compounds and compositions to treat medical disorders, e.g., Gaucher disease, Parkinson's disease, Lewy body disease, dementia, or multiple system atrophy, in a patient. Exemplary heterobicyclic pyrimidinone compounds described herein include 5-oxo- 2,3-dihydro-5H-hiazolo[3,2-a]pyrimidine-6-carboxarnide compounds.
-
Design, synthesis, biological evaluation and molecular modeling investigation of new N'-(2-Thiouracil-5-oyl) hydrazone derivatives as potential anti-breast cancer and anti-bacterial agents作者:Abd-Allah Sh. El-Etrawy、Farag F. SherbinyDOI:10.1016/j.molstruc.2021.129993日期:2021.5exhibited significant activity against Pseudomonas aeruginosa only and compound 3j displayed low activity against Staphylococcus aureus. The structure-antibacterial activity relationship analysis can be modulated by the presence of aromatic or heteroaromatic moiety containing more lipophilic character significantly contributed to antibacterial activity. In addition, the drug-likeness properties have predicted设计了一系列新颖的N '-(2-硫尿嘧啶-5-酰基)hydr,并使用活性亚结构组合法化学合成。根据元素分析(%),IR,MS,1 H NMR和13 C NMR光谱对合成的化合物进行结构表征。使用MTT测定法在体外针对MCF-7人乳腺细胞系评估了所有制备的化合物。抗癌结果表明,化合物3j,4a,3c,3b和3h对IC 50的乳腺癌细胞系表现出最显着的作用使用阿霉素作为对照药物时,其最大浓度分别为3.40、3.50、3.60、3.70和3.80 µg / ml。此外,还进行了分子对接研究,以鉴定这些化合物与预期目标胸苷酸合酶(PDB:1JU6)的结合模式机制。在另一方面,所有制备的化合物的抗菌活性进行了筛选体外对三名细菌菌株,即大肠杆菌,和铜绿假单胞菌作为革兰氏阴性菌和金黄色葡萄球菌采用琼脂孔扩散方法作为革兰氏阳性细菌。抗菌活性结果表明,大多数被测化合物均无活性,但是在所有被测化合物中,只有3g和4a,浓度为50μg/
表征谱图
-
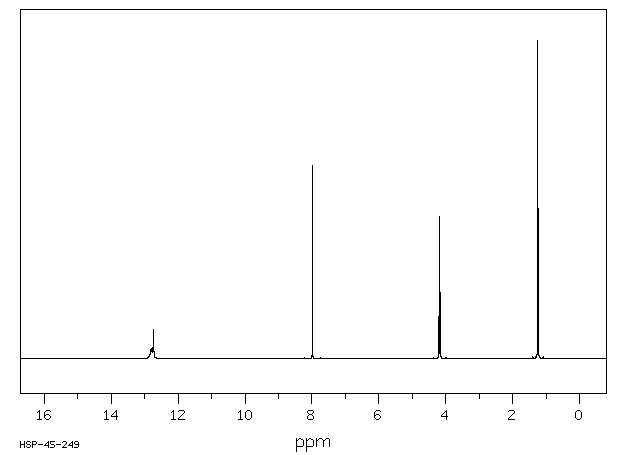
氢谱1HNMR
-
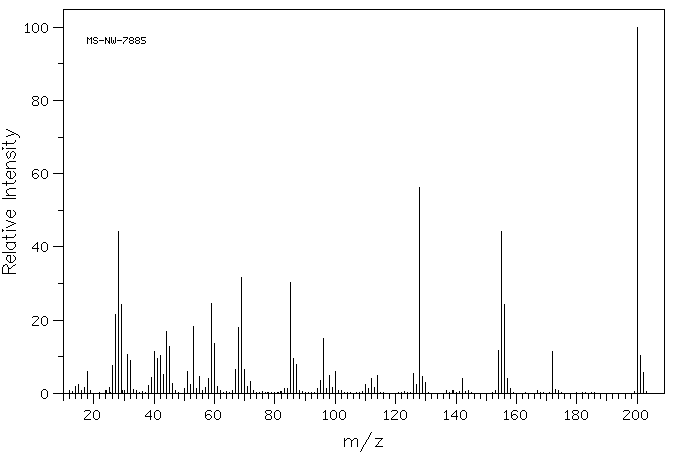
质谱MS
-
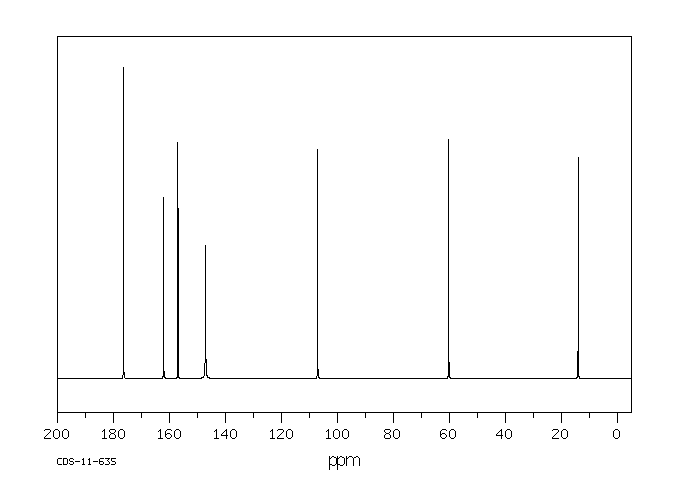
碳谱13CNMR
-
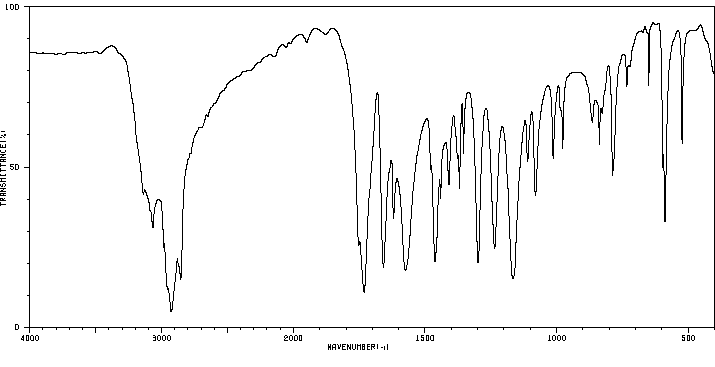
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3