5-甲基-异噻唑 | 693-97-0
中文名称
5-甲基-异噻唑
中文别名
——
英文名称
5-methylisothiazole
英文别名
5-Methyl-isothiazol;5-methyl-1,2-thiazole
CAS
693-97-0
化学式
C4H5NS
mdl
MFCD00003160
分子量
99.1564
InChiKey
LBBKWEDRPDGXPM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:145 °C
-
密度:1.101 g/cm3
-
保留指数:820
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:6
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:41.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
反应信息
-
作为反应物:描述:5-甲基-异噻唑 在 selenium(IV) oxide 作用下, 反应 3.0h, 以8%的产率得到异噻唑-5-羧酸参考文献:名称:Lucchesini, Francesco; Bertini, Vincenzo; Munno, Angela De, Heterocycles, 1985, vol. 23, # 1, p. 127 - 132摘要:DOI:
-
作为产物:参考文献:名称:Phototransposition chemistry of methylisothiazoles and methylthiazoles摘要:Methylisothiazoles undergo phototransposition in neutral solution to methylthiazoles by a single permutation process. Methylisothiazole --> methylisothiazole transpositions, previously reported to occur, were not detected in these reactions. In trifluoroacetic acid solvent, protonated methylisothiazoles and methylthiazoles phototranspose by P4 and P5 permutation pathways, respectively, resulting in a unique phototransposition cycle.DOI:10.1021/jo00064a033
文献信息
-
Muehlstaedt,M. et al., Journal fur praktische Chemie (Leipzig 1954), 1976, vol. 318, p. 507 - 514作者:Muehlstaedt,M. et al.DOI:——日期:——
-
Phase transfer catalysed reductive acylation of nitrogen-containing heteroaromatics with acetylcobalt tetracarbonyl作者:Ming de Wang、Howard AlperDOI:10.1016/0022-328x(93)83023-o日期:1993.6Phase transfer catalyzed reductive ring-cleavage acylation of isoxazoles or isothiazoles with acetylcobalt tetracarbonyl gives N-acylated 1-amino-2-alkene-3-ones or thiones. Under the same conditions phthalazine, quinoline and isoquinoline react with acetylcobalt tetracarbonyl to give N-acylated dimers. The reactivity of several other nitrogen-containing heterocycles was also investigated.
-
LUCCHESINI, FRANCESCO;PICCI, NEVIO;POCCI, MARCO;MUNNO, ANGELA DE;BERTINI,+, HETEROCYCLES, 29,(1989) N, C. 97-102作者:LUCCHESINI, FRANCESCO、PICCI, NEVIO、POCCI, MARCO、MUNNO, ANGELA DE、BERTINI,+DOI:——日期:——
-
SINHA, A. I. P.;JAIN, J. L.;SINHA, B. K., SYNTH. AND REACT. INORG. AND METAL-ORG. CHEM., 1984, 14, N 2, 151-161作者:SINHA, A. I. P.、JAIN, J. L.、SINHA, B. K.DOI:——日期:——
-
LUCCHESINI, F.;BERTINI, V.;MUNNO, A. DE, HETEROCYCLES, 1985, 23, N 1, 127-132作者:LUCCHESINI, F.、BERTINI, V.、MUNNO, A. DEDOI:——日期:——
表征谱图
-
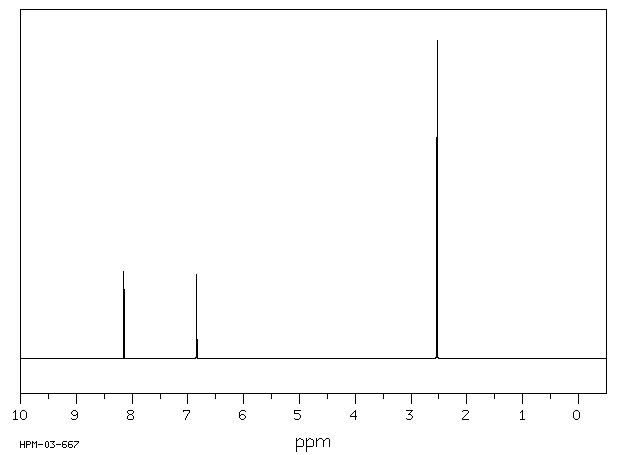
氢谱1HNMR
-
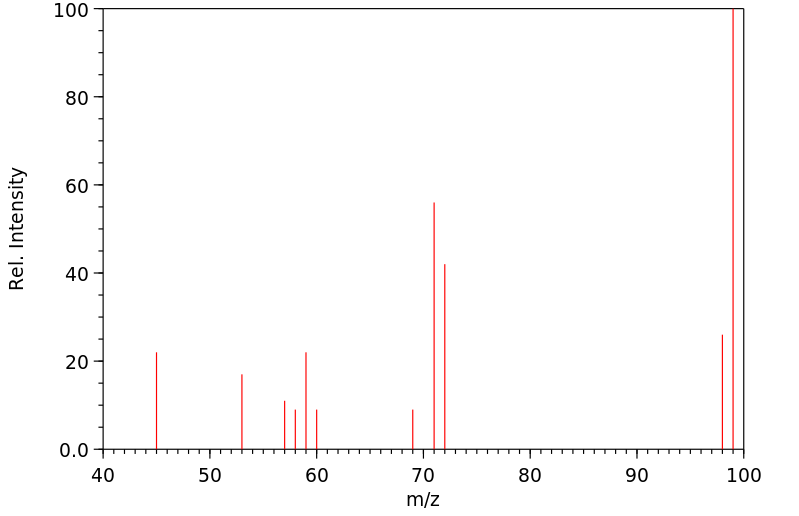
质谱MS
-
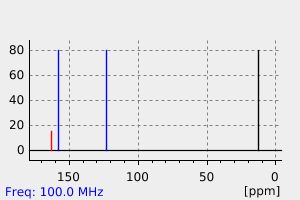
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)