5-胆甾烯 | 570-74-1
分子结构分类
中文名称
5-胆甾烯
中文别名
——
英文名称
cholest-5-ene
英文别名
5-cholestene;(8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene
CAS
570-74-1
化学式
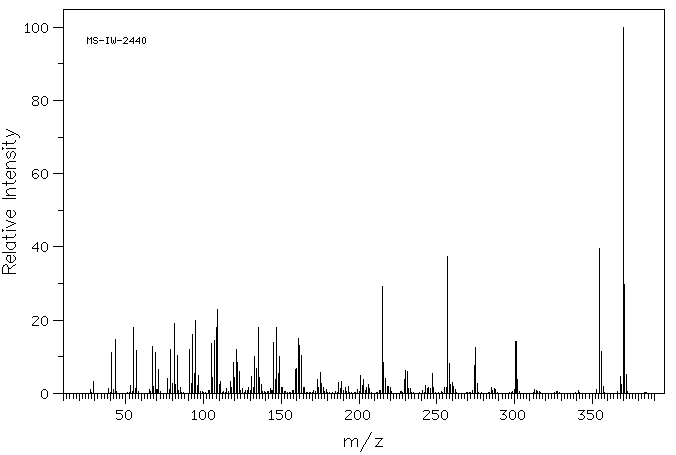
C27H46
mdl
——
分子量
370.662
InChiKey
DTGDZMYNKLTSKC-HKQCOZBKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:92.85°C
-
沸点:435.51°C (rough estimate)
-
密度:0.8753 (estimate)
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):10.1
-
重原子数:27
-
可旋转键数:5
-
环数:4.0
-
sp3杂化的碳原子比例:0.93
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 胆固醇碘化物 cholesteryl iodide 2930-80-5 C27H45I 496.559 5-胆甾烯-3-酮 Cholest-5-en-3-one 601-54-7 C27H44O 384.646 巯基胆固醇 thiocholesterol 1249-81-6 C27H46S 402.728 —— (3S)-3α-cholestanethiol 80312-90-9 C27H46S 402.728 胆甾醇溴 (3S,8S,9S,10R,13R,14S,17R)-3-Bromo-17-((R)-1,5-dimethyl-hexyl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene 516-91-6 C27H45Br 449.558 —— 3α-bromocholest-5-ene 2871-53-6 C27H45Br 449.558 氯化胆固醇 cholesteryl chloride 910-31-6 C27H45Cl 405.107 —— cholesteryl chloride 96345-96-9 C27H45Cl 405.107 —— 3-chloro-cholest-5-ene 910-31-6 C27H45Cl 405.107 胆固醇 cholesterol 57-88-5 C27H46O 386.662 胆甾-5-烯-3-醇 cholesterol 765935-41-9 C27H46O 386.662 —— cholest-5-en-7-one 22033-90-5 C27H44O 384.646 —— (25R)-cholest-5-en-3β,16β,26-triol 31327-56-7 C27H46O3 418.66 胆固醇醋酸酯 Cholesteryl acetate 604-35-3 C29H48O2 428.699 —— cholester-3β-yl thioacetate 57701-06-1 C29H48OS 444.766 胆固醇甲酰氯 Cholesteryl chloroformate 7144-08-3 C28H45ClO2 449.117 —— O-cholesteryl-S-methylxanthate —— C29H48OS2 476.832 —— O-cholesteryl-S-methyl dithiocarbonate 53496-46-1 C29H48OS2 476.832 [(3S,8S,9S,10R,13R,14S,17R)-10,13-二甲基-17-[(2R)-6-甲基庚烷-2-基]-2,3,4,7,8,9,11,12,14,15,16,17-十二氢-1H-环戊二烯并[a]菲-3-基]甲烷磺酸酯 cholesterol mesylate 3381-54-2 C28H48O3S 464.753 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 胆甾-4-烯 cholest-4-ene 16732-86-8 C27H46 370.662 —— (20R)-5β,14β-dimethyl-18-19-dinor-8α,9β,10α-cholest-13(17)-ene 28398-70-1 C27H46 370.662 —— (20S)-5β,14β-dimethyl-18,19-dinor-8α,9β,10α-cholest-13(17)ene 28398-71-2 C27H46 370.662 —— (5S,8S,9S,10R,14R)-17-((S)-1,5-Dimethyl-hexyl)-5,14-dimethyl-2,3,4,5,6,7,8,9,10,11,12,14,15,16-tetradecahydro-1H-cyclopenta[a]phenanthrene 117019-70-2 C27H46 370.662 4-胆甾烯-3-酮 Cholest-4-en-3-one 601-57-0 C27H44O 384.646 胆固醇 cholesterol 57-88-5 C27H46O 386.662 —— 1-[(6R,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,6,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-6-yl]ethanone 1231751-99-7 C29H48O 412.7 —— 1-[(6S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,6,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-6-yl]ethanone 1231752-00-3 C29H48O 412.7 —— cholest-5-en-7-one 22033-90-5 C27H44O 384.646 —— 6β-propionyl-cholest-4-ene 1231752-01-4 C30H50O 426.726 胆甾-4-烯-6-酮 cholest-4-en-6-one 13095-36-8 C27H44O 384.646 —— Cholest-4-en-3β,6β-diol 570-88-7 C27H46O2 402.661 胆固醇醋酸酯 Cholesteryl acetate 604-35-3 C29H48O2 428.699 胆甾-3,5-二烯-7-酮 cholest-3,5-dien-7-one 567-72-6 C27H42O 382.63 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Ahmad, M. S.; Ayad, Tariq M., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1991, vol. 30, # 8, p. 763 - 768摘要:DOI:
-
作为产物:描述:胆固醇 在 咪唑 、 碘 、 三苯基膦 、 fac-tris(2-phenylpyridinato-N,C2')iridium(III) 、 N,N-二异丙基乙胺 作用下, 以 甲苯 、 乙腈 、 甲醇 为溶剂, 反应 0.63h, 以72%的产率得到5-胆甾烯参考文献:名称:使用可见光光氧化还原催化间歇流脱氧。摘要:本文中,我们报告了通过Garegg-Samuelsson反应,可见光-光氧化还原催化和流化法相结合开发的伯醇和仲醇的一锅脱氧方案。该方法的特点是反应条件温和,反应物和试剂易于处理,对官能团的耐受性强,产率高。DOI:10.1039/c2cc37206a
文献信息
-
Studies of the synthesis of 5-hydroxy 6-keto steroids and related 6-keto steroids作者:Peter Yates、Shirley StiverDOI:10.1139/v87-369日期:1987.9.1
Syntheses of 5-hydroxy-5α- and 5β-cholestan-6-one (11 and 13) and their 3β-acetoxy (10 and 21) and 3β-benzyloxy derivatives (12 and 19) are described, as are syntheses of the 7α-deutero derivatives of 10 and 21. Related investigations of the syntheses of the 5-methoxy and 5-methyl analogues of these compounds are also discussed. Treatment of 12 with potassium tert-butoxide has been shown to give 5-hydroxy-5β-cholest-3-en-6-one (14) and its Δ2 isomer 15. Reaction of 6-nitrocholesteryl acetate (50) with lithium dimethylcuprate gives 3α,5-cyclo-5α-cholestan-6-one (E)-oxime (51) as the major product.
-
Regioselective transformation of allylic, benzylic and tertiary alcohols into the corresponding iodides wrih aluminium triiodide: deoxygenation of vicinal diols作者:Parijat Sarmah、Nabin C BaruaDOI:10.1016/s0040-4020(01)81035-8日期:1989.1Aluminium triiodide regioselectively converts allylic, benzylic and tertiary alcohols Into the corresponding iodides in excellent yields. It also deoxygenates cis and trans secondary-tertiary and allylic bis—secondary vicinal diols in good yields.
-
Tris(3,6-dioxaheptyl)amine: A superior complexing agent for dissolving metal reactions作者:Ajay K. Bose、Pietro MangiaracinaDOI:10.1016/s0040-4039(00)95452-2日期:1987.1Commercially available tris(3,6-dioxaheptyl)amine (TDA-1) is an effective phase transfer catalyst for reactions with sodium-potassium alloy in tetrahydrofuran. Deoxygenation of acetates, dehalogenation, and hydrolysis of tosylates and mesylates proceed in high yield. The use of a deuterated alcohol during deoxygenation reactions leads to site-specific deuteration.
-
Synthesis and<i>In Vitro</i>Antibacterial Activity of Novel Steroidal (6R)-Spiro-1,3,4-thiadiazoline Derivatives作者:Salman A. Khan、Abdullah M. AsiriDOI:10.1002/jhet.1014日期:2012.11Novel steroidal (6R)‐spiro‐1,3,4‐thiadiazoline derivatives were synthesized by the cyclization of steroidal thiosemicarbazones with acetic anhydride, screened in vitro against antibacterial activity using disc‐diffusion method and the minimum inhibitory concentration. The results showed that steroidal thiadiazoline derivatives exhibited better antibacterial activity than the steroidal thiosemicarbazone
-
A radical deoxygenation of secondary alcohols to hydrocarbons by use of tributyltin hydride作者:Yoshihiko Watanabe、Takumi Araki、Yoshio Ueno、Takeshi EndoDOI:10.1016/s0040-4039(00)85218-1日期:1986.1Secondary alcohols reacted with 2,2′-dibenzothiazolyl- disulfide in the presence of tributylphosphine to give corresponding sulfides in good yields. Sulfides were subsequently desulfurized to hydrocarbons with tributyltin hydride in radical conditions.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
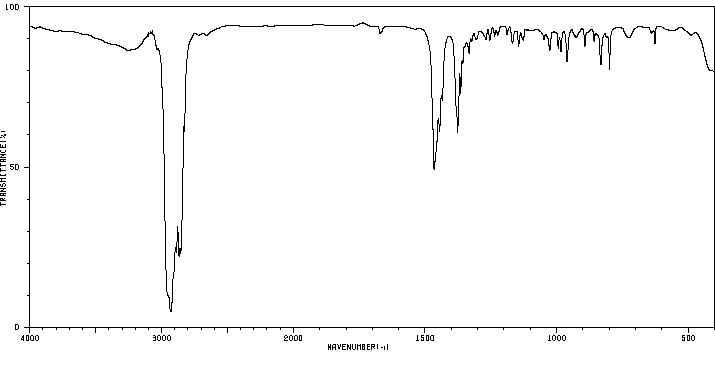
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B