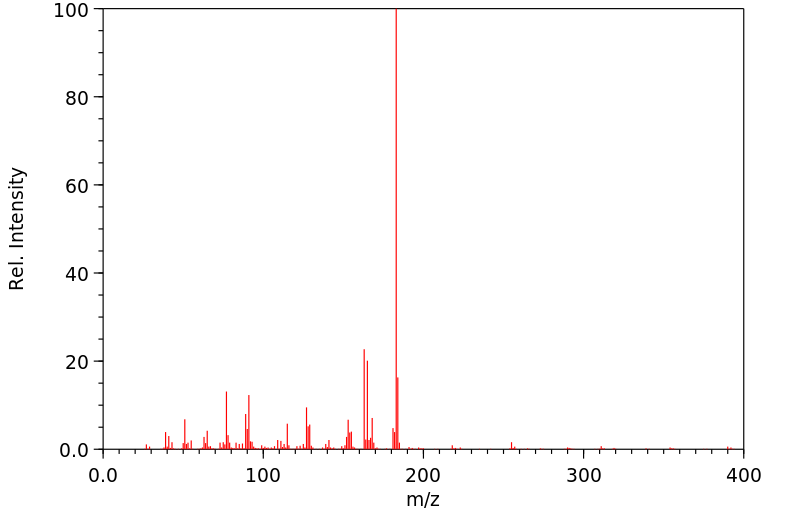
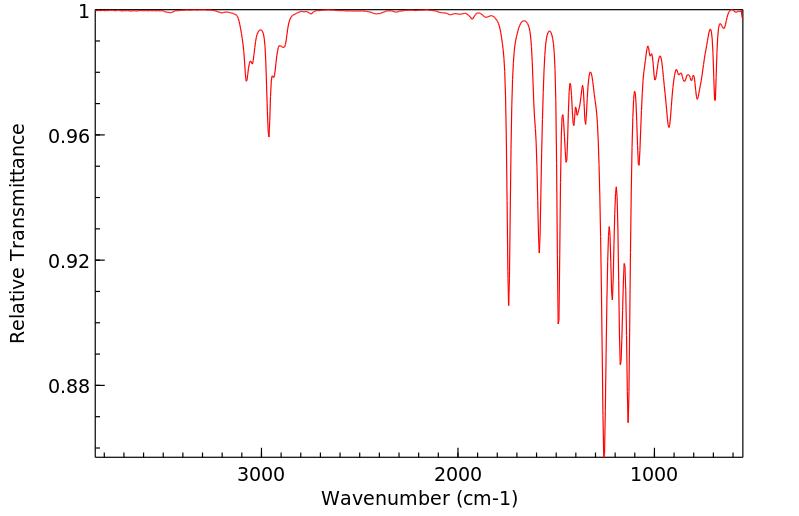
顺式氯菊酯 | 61949-76-6
中文名称
顺式氯菊酯
中文别名
——
英文名称
Eksmin
英文别名
(3-phenoxyphenyl)methyl (1R,3R)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate;cis-(-)-3'-phenoxybenzyl-3-(2',2'-dichlorovinyl)-2,2-dimethyl-cyclopropane carboxylate;3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate;14C-cis-permethrin;1R-(-)-cis-permethrin;(R)-cis-permethrin;cis-Permethrin;(3-phenoxyphenyl)methyl (1R,3R)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate
CAS
61949-76-6;54774-45-7
化学式
C21H20Cl2O3
mdl
——
分子量
391.294
InChiKey
RLLPVAHGXHCWKJ-HKUYNNGSSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:63~65℃
-
沸点:505.74°C (rough estimate)
-
密度:1.1531 (rough estimate)
-
闪点:-18 °C
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
物理描述:YELLOW-BROWN-TO-BROWN VISCOUS LIQUID OR CRYSTALS.
-
蒸汽压力:Vapor pressure, Pa at 20 °C:
-
保留指数:2615;2662;2616.5;2609.3;2636.7;2615
计算性质
-
辛醇/水分配系数(LogP):6.5
-
重原子数:26
-
可旋转键数:7
-
环数:3.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xn,F,N
-
安全说明:S13,S16,S24,S36/37/39,S60,S61,S62
-
危险类别码:R67,R38,R43,R20/22,R50/53,R11,R65
-
WGK Germany:2,3
-
海关编码:2916209090
-
危险品运输编号:UN 1145 3/PG 2
SDS
制备方法与用途
用途
顺式-氯菊酯标准溶液主要应用于农药残留分析、环境监测及质量控制等领域。作为一种分析标准,它能够确保实验结果的准确性和可靠性。
反应信息
-
作为反应物:描述:顺式氯菊酯 、 chlorozinc(1+),2H-furan-2-ide 以87%的产率得到参考文献:名称:MINATO, AKIO, J. ORG. CHEM., 56,(1991) N2, C. 4052-4056摘要:DOI:
-
作为产物:描述:4,7,7-trimethylbicyclo[4.1.0]hept-3-en-2-one 在 吡啶 、 chromium(VI) oxide 、 盐酸 、 六甲基磷酰三胺 、 sodium hydroxide 、 potassium permanganate 、 三乙胺 、 3-氯苯甲酸 作用下, 以 二氯甲烷 、 水 、 溶剂黄146 、 正戊烷 为溶剂, 反应 97.0h, 生成 顺式氯菊酯参考文献:名称:从(+)-3-care合成(1r)-顺-(-)-氯菊酯,(1r)-顺-(+)-氯氰菊酯和(1r)-顺-(+)-溴氰菊酯(癸)的新合成路线摘要:从(+)-3-carene(5)容易获得的(+)-4α-乙酰基-2-carene(6)被转化为(1R)-cis-(+)-3-(2',2 (11)和(22)的11个步骤中的'-(二卤代戊酰基)-2、2-二甲基-环丙烷-1-羧酸(21)和(22)的总收率分别为23%和14%。或者,将(+)-3-烯烃(5)的氧化产物(-)-5-酮-3-烯烃(23)在五次转化为(1R)-顺式-(-)-氯菊酯(1)步骤,总收率为20%。在另一种灵活的方法中,将(-)-(23)分为七个步骤,整体上分别转换为(1R)-顺式-(+)-(21)和(1R)-顺式-(+)-(22)产率分别为33%和23%。这些结果与文献报道有关将(1R)-顺式-(+)-(21)和(22)转化为(1R)-顺式-(-)-(1),DOI:10.1016/s0040-4020(01)88177-1
文献信息
-
Assignment of absolute configurations of permethrin and its synthon 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid by electronic circular dichroism, optical rotation, and X-ray crystallography作者:Wolfgang Bicker、Karol Kacprzak、Marcin Kwit、Michael Lämmerhofer、Jacek Gawronski、Wolfgang LindnerDOI:10.1016/j.tetasy.2009.03.035日期:2009.53-phenoxybenzyl alcohol yielded PM. Electronic circular dichroism (ECD) spectra of DCCA and PM stereoisomers were measured in non-polar (cyclohexane containing 5% v/v 1,2-dichloroethane) and non-protic polar (acetonitrile) solvents. Cotton effects suitable to distinguish the four stereoisomers of each DCCA and PM were obtained. Absolute configurations of DCCA were determined by confrontation of calculated生物/毒理学相关手性化合物(如拟除虫菊酯型杀虫剂氯菊酯(PM))的单一立体异构体的可用性以及其绝对构型的可靠测定对于详细研究和正确选择立体选择效应至关重要。在这种情况下,从所有四种立体异构体的对映异构体混合物中分离出3-(2,2-二氯乙烯基)-2,2-二甲基环丙烷羧酸(DCCA),PM的前体,代谢产物和环境降解产物的单一立体异构体通过第一步色谱中的非对映选择性反相分离与第二步中的直接对映异构体分离相结合的两步色谱法,获得了> 99%的过量残留物。用3-苯氧基苄醇酯化DCCA立体异构体得到PM。在非极性(含有5%v / v 1,2-二氯乙烷的环己烷)和非质子极性(乙腈)溶剂中测量DCCA和PM立体异构体的电子圆二色性(ECD)光谱。获得了适合区分每种DCCA和PM的四种立体异构体的棉花效应。DCCA的绝对构型由计算值(使用B3LYP混合函数的时变密度泛函理论)和实验ECD和旋光(OR)数据的
-
High concentration topical insecticides containing pyrethroids申请人:Cottrell W. Ian公开号:US20050245582A1公开(公告)日:2005-11-03A topical insecticide preparation is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. The topical insecticide contains a combination of a first pyrethroid insecticide effective for killing fleas, a second pyrethroid insecticide effective for killing ticks, and an insect growth regulator (IGR). The topical insecticide preparation can be packaged together or packaged so that the first and second pyrethroid insecticides are stored separately prior to administration of the insecticide preparation to the animal. The combination of the first and second pyrethroid insecticides with an insect growth regulator results in an insecticide preparation formulated to have enhanced insecticidal activity against fleas and ticks compared to the effectiveness of the first and second insecticides used alone. Further, the combination of the first and second pyrethroid insecticides with an insect growth regulator produces an insecticide preparation having enhanced insecticidal activity against fleas and ticks while advantageously minimizing the total amount of insecticide needed for its effectiveness.
-
HIGH CONCENTRATION TOPICAL INSECTICIES CONTAINING PYRETHROIDS申请人:Cottrell Ian公开号:US20070276014A1公开(公告)日:2007-11-29A topical insecticide preparation is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. The topical insecticide contains a combination of a first pyrethroid insecticide effective for killing fleas, a second pyrethroid insecticide effective for killing ticks, and an insect growth regulator (IGR). The topical insecticide preparation can be packaged together or packaged so that the first and second pyrethroid insecticides are stored separately prior to administration of the insecticide preparation to the animal. The combination of the first and second pyrethroid insecticides with an insect growth regulator results in an insecticide preparation formulated to have enhanced insecticidal activity against fleas and ticks compared to the effectiveness of the first and second insecticides used alone. Further, the combination of the first and second pyrethroid insecticides with an insect growth regulator produces an insecticide preparation having enhanced insecticidal activity against fleas and ticks while advantageously minimizing the total amount of insecticide needed for its effectiveness.
-
HIGH CONCENTRATION TOPICAL INSECTICIDES CONTAINING PYRETHROIDS申请人:Cottrell Ian公开号:US20110144166A1公开(公告)日:2011-06-16A topical insecticide preparation is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. The topical insecticide contains a combination of a first pyrethroid insecticide effective for killing fleas, a second pyrethroid insecticide effective for killing ticks, and an insect growth regulator (IGR). The topical insecticide preparation can be packaged together or packaged so that the first and second pyrethroid insecticides are stored separately prior to administration of the insecticide preparation to the animal. The combination of the first and second pyrethroid insecticides with an insect growth regulator results in an insecticide preparation formulated to have enhanced insecticidal activity against fleas and ticks compared to the effectiveness of the first and second insecticides used alone. Further, the combination of the first and second pyrethroid insecticides with an insect growth regulator produces an insecticide preparation having enhanced insecticidal activity against fleas and ticks while advantageously minimizing the total amount of insecticide needed for its effectiveness.
-
PROCESS FOR PREPARATION OF STABLE, MICROENCAPSULATED AND SUSTAINED RELEASE BIOCIDAL ACTIVES AND COMPOSITION THEREOF申请人:Premachandran Raman公开号:US20120164203A1公开(公告)日:2012-06-28Disclosed herein is a process for the preparation of a stable sustained-release biocidal composition containing microencapsulated biocide and wherein the process comprises the steps of: (i) adsorbing the biocide onto an inert carrier by grinding to attain the required particle size and wherein the ratio of biocide; inert carrier is in the range of about 1:99 to about 99:1 (ii) optionally coating with an appropriate amine or imine compound or a water resistant film forming polymer and dispersing the resultant biocide encapsulated inert carrier in an aqueous medium in the presence of suitable dispersing agent (iii) adding at least one thickening agent to re-disperse the encapsulated biocide containing partial amount of non-encapsulated biocide if any and (iv) preparing an aqueous or solvent based sustained release biocide dispersion. Also disclosed is a stable, sustained-release biocidal composition prepared by such process and uses thereof.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯