6-溴甲基-2-吡啶甲醇 | 40054-01-1
中文名称
6-溴甲基-2-吡啶甲醇
中文别名
2-(溴甲基)-6-(羟甲基)吡啶;2-溴甲基-6-羟基甲基吡啶;6-(溴甲基)-2-吡啶甲醇
英文名称
(6-(bromomethyl)pyridin-2-yl)methanol
英文别名
6-(bromomethyl)-2-pyridinemethanol;6-(bromomethyl)-2-(hydroxymethyl)pyridine;[6-(bromomethyl)pyridin-2-yl]methanol
CAS
40054-01-1
化学式
C7H8BrNO
mdl
——
分子量
202.051
InChiKey
JLYYQZROGJLCCS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:80 °C
-
沸点:284.1±30.0 °C(Predicted)
-
密度:1.604±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:33.1
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2933399090
-
包装等级:II
-
危险类别:8
-
危险性防范说明:P260,P264,P270,P280,P301+P330+P331,P303+P361+P353,P304+P340,P305+P351+P338,P310,P363,P405,P501
-
危险品运输编号:3261
-
危险性描述:H302,H314
-
储存条件:存放于室温且保存在惰性气体中
SDS
6-(Bromomethyl)-2-pyridinemethanol Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 6-(Bromomethyl)-2-pyridinemethanol
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 6-(Bromomethyl)-2-pyridinemethanol
Percent: >98.0%(GC)(T)
CAS Number: 40054-01-1
Synonyms: 2-(Bromomethyl)-6-(hydroxymethyl)pyridine
Chemical Formula: C7H8BrNO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
6-(Bromomethyl)-2-pyridinemethanol
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Slightly pale yellow red
Odor: No data available
pH: No data available
6-(Bromomethyl)-2-pyridinemethanol
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Melting point/freezing point:80 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
6-(Bromomethyl)-2-pyridinemethanol
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 6-(Bromomethyl)-2-pyridinemethanol
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 6-(Bromomethyl)-2-pyridinemethanol
Percent: >98.0%(GC)(T)
CAS Number: 40054-01-1
Synonyms: 2-(Bromomethyl)-6-(hydroxymethyl)pyridine
Chemical Formula: C7H8BrNO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
6-(Bromomethyl)-2-pyridinemethanol
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Slightly pale yellow red
Odor: No data available
pH: No data available
6-(Bromomethyl)-2-pyridinemethanol
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Melting point/freezing point:80 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
6-(Bromomethyl)-2-pyridinemethanol
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
概述
6-溴甲基-2-吡啶甲醇是一种醇类衍生物,可通过将2,6-吡啶基甲醇进行溴代反应得到。
用途6-溴甲基-2-吡啶甲醇同样作为醇类衍生物,可用作有机中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,6-吡啶二甲醇 2,6-Pyridinedimethanol 1195-59-1 C7H9NO2 139.154 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (6-(氨基甲基)吡啶-2-基)甲醇 6-aminomethyl-2-hydroxymethylpyridine 50501-31-0 C7H10N2O 138.169 —— 2-(bromomethyl)-6-(((tetrahydro-2H-pyran-2-yl)oxy)methyl)pyridine 162067-24-5 C12H16BrNO2 286.169
反应信息
-
作为反应物:描述:6-溴甲基-2-吡啶甲醇 在 硫酸 、 potassium carbonate 作用下, 以 乙腈 为溶剂, 反应 9.0h, 生成 [6-{[(pyrid-2-ylmethyl)amino]methyl}pyrid-2-yl]methanol参考文献:名称:Syntheses of Zinc Complexes with Multidentate Nitrogen Ligands: New Catalysts for Aldol Reactions摘要:DOI:10.1002/1099-0682(200212)2002:12<3284::aid-ejic3284>3.0.co;2-6
-
作为产物:描述:参考文献:名称:含二吡啶的大环三酰胺 Fe(II) 和 Ni(II) 配合物作为 ParaCEST 试剂摘要:Fe(II) 和 Ni(II) paraCEST 造影剂含有二吡啶大环配体 2,2',2"-(3,7,10-triaza-1,5(2,6)-dipyridinacycloundecaphane-3,7本文报道了 ,10-三基)三乙酰胺 (DETA)。[Fe(DETA)] 2+和[Ni(DETA)] 2+配合物都进行了结构表征。晶体学数据揭示了[Fe(DETA)]·(BF 4 ) 2 ·MeCN 配合物的七配位扭曲五角双锥几何结构,其中五个配位氮原子来自大环,两个配位氧原子来自两个酰胺侧臂。[Ni(DETA)]·Cl 2 ·2H 2O 复合体本质上是六配位的,具有扭曲的八面体几何形状。四个配位的氮原子来自大环,两个配位的氧原子来自两个酰胺侧臂。[Fe(DETA)] 2+表现出很好分辨的尖锐质子共振,而在[Ni(DETA)] 2+的情况下,由于电子弛豫时间长,观察到非常宽的质子共振。[Fe(DETA)]DOI:10.1021/acs.inorgchem.2c02242
文献信息
-
SNS-Ligands for Ru-Catalyzed Homogeneous Hydrogenation and Dehydrogenation Reactions作者:Johannes Schörgenhumer、Axel Zimmermann、Mario WaserDOI:10.1021/acs.oprd.8b00142日期:2018.7.20literature-known and novel S-containing pincer-type ligands for ruthenium-catalyzed homogeneous hydrogenation and dehydrogenation reactions was carried out. The scope and limitations of these catalysts were carefully investigated, and it was shown that simple bench-stable SNS–Ru complexes can be used to facilitate the hydrogenation of a variety of different substrates at a maximum H2 pressure of 20 bar under operationally
-
Synthesis of Polyaza Macrocyclic Ligands Incorporating Pyridine Units作者:Mir Wais Hosseini、Jacques Comarmond、Jean-Marie LehnDOI:10.1002/hlca.19890720525日期:1989.8.9The synthesis of nine macrocyclic polyamines 2-10 containing pyridine units is described. These compounds are 22- (9), 24- (2-4,6,8,10), or 26- (5, 7) membered hexaaza (2,3,9,10) or octaaza (4–7) macrocycles in which one to four pyridine units are incorporated. Compounds 3,4,9, and 10 are homoditopic ligands, whereas 2 and 5 are heteroditopic and 6−8 multitopic receptors. Compounds 2-10 are potential
-
Compounds having a plurality of nitrogenous substitutents申请人:ISIS Pharmaceuticals, Inc.公开号:US06329523B1公开(公告)日:2001-12-11Novel compounds having the formula: wherein the constituent variables are defined herein. The compounds are constructed to include a central aromatic, aliphatic, or heterocyclic ring system. Attached to the central ring system are two linear groups having nitrogenous moieties that are derivatized with chemical functional groups. The ring system can include further nitrogenous moieties, either as ring atoms or on pendant groups attached to the ring, that may also be derivatized with chemical functional groups. The totality of the chemical functional groups imparts certain conformational and other properties to the these compounds. In accordance with certain embodiments of the invention, libraries of such compounds are prepared utilizing permutations and combinations of the chemical functional groups and the nitrogenous moieties to build complexity into the libraries.
-
Identification of human T2R receptors that respond to bitter compounds that elicit the bitter taste in compositions, and the use thereof in assays to identify compounds that inhibit (block) bitter taste in composition and use thereof申请人:Li Xiaodong公开号:US20090274632A1公开(公告)日:2009-11-05The present invention relates to the discovery that specific human taste receptors in the T2R taste receptor family respond to particular bitter compounds present in, e.g., coffee. Also, the invention relates to the discovery of specific compounds and compositions containing that function as bitter taste blockers and the use thereof as bitter taste blockers or flavor modulators in, e.g., coffee and coffee flavored foods, beverages and medicaments. Also, the present invention relates to the discovery of a compound that antagonizes numerous different human T2Rs and the use thereof in assays and as a bitter taste blocker in compositions for ingestion by humans and animals.本发明涉及发现T2R味觉受体家族中特定人类味觉受体对咖啡等中特定苦味化合物的反应。此外,本发明涉及发现特定化合物和含有该化合物的组合物作为苦味阻断剂的功能,以及将其用作苦味阻断剂或调味剂,例如在咖啡和咖啡味食品、饮料和药品中。此外,本发明涉及发现一种对抗多种不同人类T2R的化合物,并将其用于检测中以及作为人类和动物摄入的组合物中的苦味阻断剂。
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
表征谱图
-
氢谱1HNMR
-
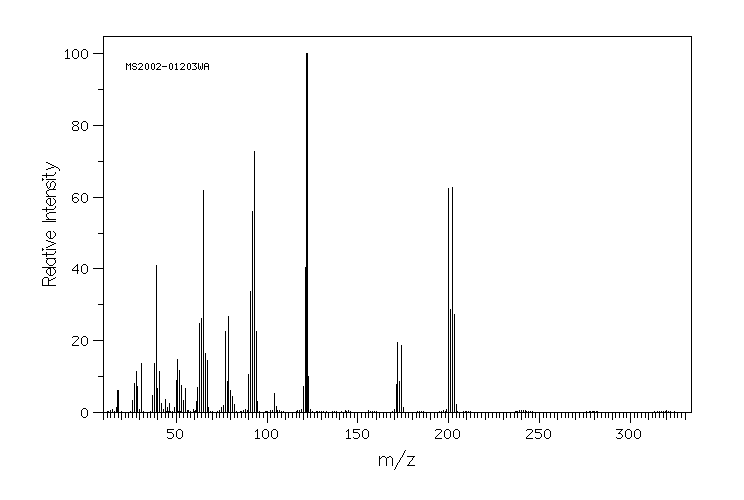
质谱MS
-
碳谱13CNMR
-
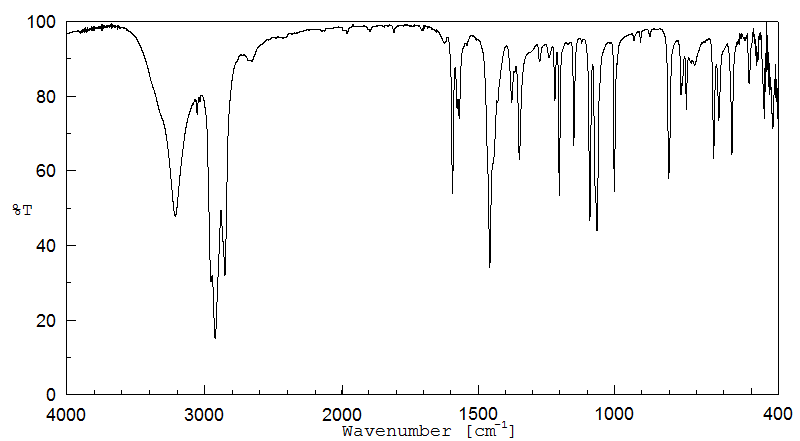
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-