苯并[b]萘并[2,3-d]呋喃 | 243-42-5
中文名称
苯并[b]萘并[2,3-d]呋喃
中文别名
——
英文名称
β-brazan
英文别名
benzo[b]naphtho[2,3-d]furan;naphtho[2,3-b]benzofuran;benzonaphtho<2,3-d>furan;naphtho[2,3-b][1]benzofuran
CAS
243-42-5
化学式
C16H10O
mdl
MFCD00022295
分子量
218.255
InChiKey
FTMRMQALUDDFQO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:202-207 °C
-
沸点:318.94°C (rough estimate)
-
密度:1.0826 (rough estimate)
-
保留指数:2089;2104
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:17
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:13.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S36/37
-
危险类别码:R40
-
海关编码:2932999099
-
储存条件:密封储存,宜存放在阴凉、干燥的库房中。
SDS
| Name: | Benzo[b]naphtho[2 3-d]furan tech. 90+% (gc) Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 243-42-5 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 243-42-5 | Benzo[b!naphtho[2,3-d!furan | 90.0 | 205-955-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Cancer suspect agent.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
May cause cancer in humans.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 243-42-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: pale yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 203.00 - 206.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C16H10O
Molecular Weight: 218.25
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 243-42-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Benzo[b!naphtho[2,3-d!furan - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 243-42-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 243-42-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 243-42-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3-苯并呋喃 1-benzofurane 271-89-6 C8H6O 118.135
反应信息
-
作为反应物:描述:参考文献:名称:함산소 축합환 아민 화합물, 함황 축합환 아민 화합물 및 유기 전기발광 소자摘要:这段文字是关于化学领域的内容,描述了含有퓨란환或싸이오펜환的化合物的化学式(1),以及包括阴极、阳极、以及嵌入在阴极和阳极之间的单层或多层有机薄膜层,其中该有机薄膜层包含发光层,并且该有机薄膜层的至少一层包含所述化合物的有机电致发光器件。文中还包括了几个化学式和结构的描述,涉及到不同元素和基团的结构。公开号:KR20150098631A
-
作为产物:描述:3-(2-溴苯基)萘-2-醇 在 potassium carbonate 、 copper(II) oxide 作用下, 以 吡啶 为溶剂, 反应 4.0h, 以73%的产率得到苯并[b]萘并[2,3-d]呋喃参考文献:名称:新的“ 2-苯基萘”介导的苯并[ b ]萘[2,3 - d ]呋喃-6,11-二酮和6-氧杂苯并[ a ]蒽-5,7,12-三酮的合成合成6-氧杂苯并[ a ]蒽-5-酮摘要:我们在这里描述基于2-(2-溴苯基)-3-羟基-1,4-萘醌杂环化的苯并[ b ]萘并[2,3 - d ]呋喃-6,11-二酮的新型合成方法。萘醌由3-(2-溴苯基)萘-2-醇制备,它们是通过2- [3-(2-溴苯基)-2-氧代丙基]苯甲醛的分子内醇醛缩合反应获得的。或者,通过将3-(2-溴苯基)萘-2-醇环化为苯并[ b ]萘[2] ,可以更直接,更有效地获得苯并[ b ]萘[2,3 - d ]呋喃-6,11-二酮。,3- d ]呋喃和所得化合物的氧化。此外,第一个6-氧杂苯并[ a]从2- [3-(2-甲酰基苯基)-2-氧丙基]苯甲酸类似地获得所述的蒽蒽-5-酮,并将其氧化成6-氧杂苯并[ a ]蒽-5,7,12-三酮。DOI:10.1016/j.tet.2004.10.044
文献信息
-
[EN] MATERIALS FOR ORGANIC ELECTROLUMINESCENT DEVICES<br/>[FR] MATÉRIAUX POUR DISPOSITIFS ÉLECTROLUMINESCENTS ORGANIQUES申请人:MERCK PATENT GMBH公开号:WO2018091435A1公开(公告)日:2018-05-24The present invention relates to compounds of the formula (1) which are suitable for use in electronic devices, in particular organic electroluminescent devices, and to electronic devices, which comprise these compounds.本发明涉及适用于电子设备,特别是有机电致发光器件的化合物的公式(1),以及包含这些化合物的电子设备。
-
NOVEL COMPOUND AND ORGANIC ELECTROLUMINESCENCE DEVICE申请人:IDEMITSU KOSAN CO.,LTD.公开号:US20200377513A1公开(公告)日:2020-12-03A compound represented by the following formula (1):以下公式(1)所代表的化合物:
-
Organic electroluminescence device and novel compound申请人:IDEMITSU KOSAN CO., LTD.公开号:US10249832B1公开(公告)日:2019-04-02To provide an organic electroluminescence device having a high luminous efficiency and a novel compound that can be used as a material for an organic electroluminescence device having a high luminous efficiency. A compound represented by the following formula (3-I), wherein at least one of R1 to R7 and R10 to R11 is —N(R36)(R37). R31 to R37 are independently a hydrogen atom, a substituted or unsubstituted alkyl group including 1 to 50 carbon atoms, a substituted or unsubstituted cycloalkyl group including 3 to 50 ring carbon atoms, a substituted or unsubstituted aryl group including 6 to 50 ring carbon atoms or a substituted or unsubstituted monovalent heterocyclic group including 5 to 50 ring atoms.提供一种具有高发光效率的有机电致发光器件和一种可用作具有高发光效率的有机电致发光器件材料的新化合物。 以下式(3-I)所代表的化合物,其中R1至R7和R10至R11中的至少一个是—N(R36)(R37)。R31至R37独立地是氢原子,包括1至50个碳原子的取代或未取代的烷基基团,包括3至50个环碳原子的取代或未取代的环烷基基团,包括6至50个环碳原子的取代或未取代的芳基基团或包括5至50个环原子的取代或未取代的单价杂环基团。
-
Palladium-Catalyzed Intramolecular C–H Arylation of Arenes Using Tosylates and Mesylates as Electrophiles作者:Christine S. Nervig、Peter J. Waller、Dipannita KalyaniDOI:10.1021/ol302166n日期:2012.9.21paper describes a method for the palladium catalyzed intramolecular C–H arylation using tosylates and mesylates as electrophiles. The transformation is efficient for the synthesis of various heterocyclic motifs including furans, carbazoles, indoles, and lactams. Additionally, a protocol for the one-pot sequential tosylation/arylation of phenol derivatives is presented.
-
Synthesis of polycyclic xanthenes and furans via palladium-catalyzed cyclization of polycyclic aryltriflate esters作者:Ji-Quan Wang、Ronald G HarveyDOI:10.1016/s0040-4020(02)00534-3日期:2002.7Palladium-catalyzed cyclization of polycyclic aromatic o-(arylmethyl)phenol triflate esters takes place with unexpected sulfur–oxygen bond cleavage to furnish polycyclic xanthenes. These are the first examples of Pd-catalyzed cross-coupling of aryl triflate esters with arenes to form diaryl ethers. In contrast, analogous palladium-catalyzed cyclization of polycyclic o-(aryloxy)phenol triflate esters
表征谱图
-
氢谱1HNMR
-
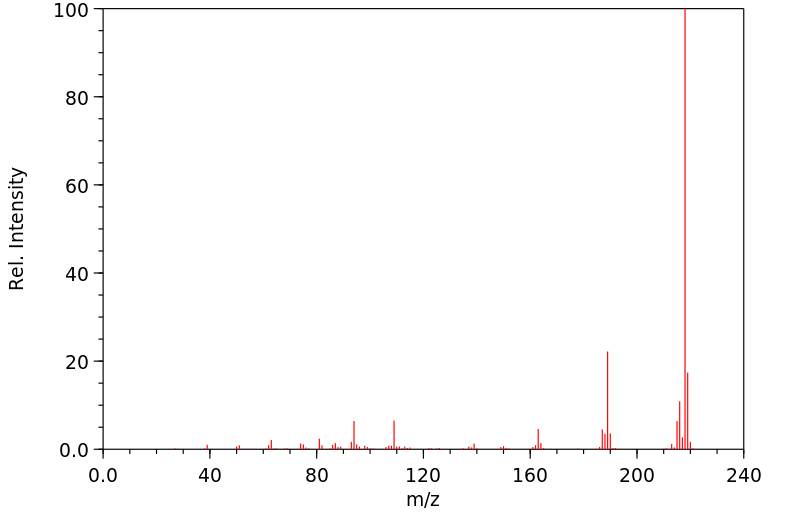
质谱MS
-
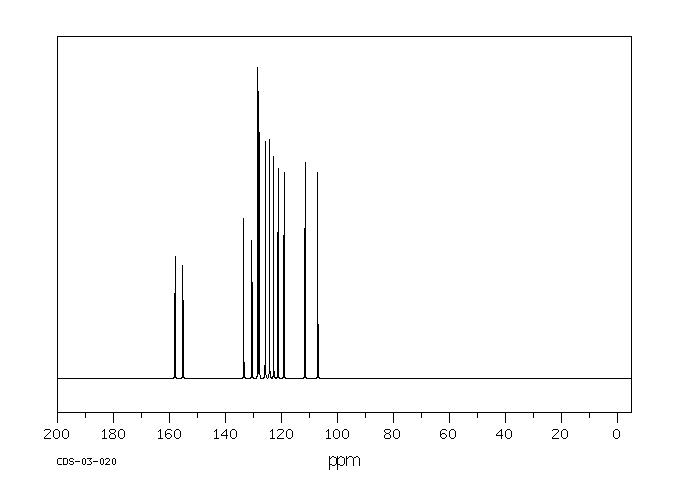
碳谱13CNMR
-
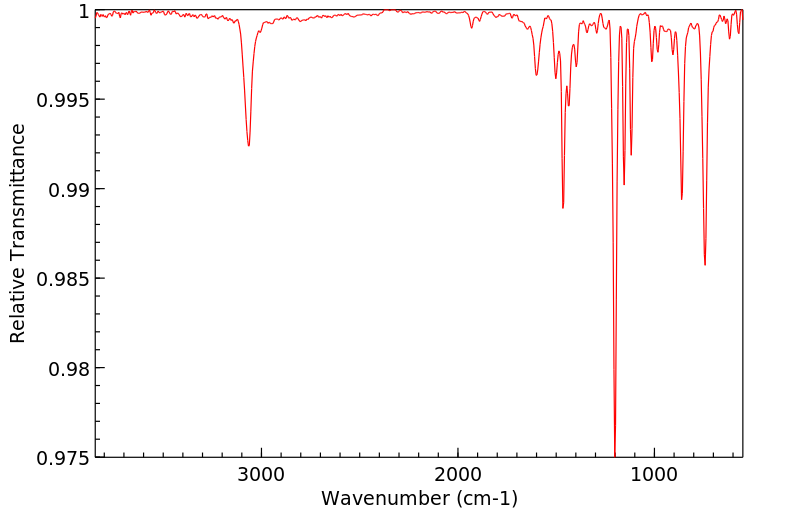
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄药子素C
黄独素A
香紫苏内酯
降龙涎香醚
阿霉素(α-β混合物)
银线草内酯醇
辛辣木素
载脂蛋白-土霉素
萘并[2,3-c]呋喃-3(1H)-酮
萘并[2,3-c]呋喃-1,3-二酮,5,8-二甲基-(9CI)
萘并[2,3-c]呋喃-1(3H)-酮,4-(3-呋喃基)-7-羟基-
萘并[2,3-b]呋喃-4,9-二酮,2,3-二氢-2-甲基-2-苯基-
萘并[2,1-b]呋喃-2-甲酰肼
萘并[2,1-b]呋喃-2(1H)-酮
萘并[2,1-b]呋喃-1-乙酸
萘并[1,2-b]呋喃-2-醇,2,3,3a,4,5,5a,6,7,9a,9b-十氢-3,5a,9-三甲基-
萘并[1,2-b]呋喃-2(3H)-酮,3a,4,5,9b-四氢-8-羟基-3,9-二甲基-,(3R,3aR,9bS)-rel-
萘并(2,3-b)呋喃-4,9-二酮
萘[2,1-b]呋喃-2-羧酸乙酯
萘[2,1-B]苯并呋喃-10-基硼酸
荧蒽-2,3-二甲酸酐
苯并[g][1]苯并呋喃-8,9-二酮
苯并[g][1]苯并呋喃-3-酮
苯并[g][1]苯并呋喃-2-甲醛
苯并[g][1]苯并呋喃
苯并[f][1]苯并呋喃-3-酮
苯并[e][1]苯并呋喃-8-醇
苯并[e][1]苯并呋喃-1-酮
苯并[e][1]苯并呋喃
苯并[b]萘并[2,3-d]呋喃
苯并[b]萘并[2,1-d]呋喃
苯并[b]萘并[1,2-d]呋喃
苯并[B]萘并[2,3-D]呋喃-2-羟基硼酸
苯基利福平
苯基(6,7,8,9-四氢萘并[2,1-b]呋喃-2-基)甲醇
苊并[5,4-b]呋喃-4,5-二酮,7,8-二氢-3,6-二羟基-1,7,7,8-四甲基-,(8S)-
维生素K1相关化合物
红葱酚
盐(1:2)苯磺酸,2,2'-(1,2-乙烯二基)二[5-[[4,6-二(2-萘氧基)-2-嘧啶基]氨基]-,钠
白术内酯 I
珀勒内B
珀勒内A
沃拉帕沙杂质
沃拉帕沙
沃拉帕沙
沃拉帕沙
己二酸,聚合2,2-二(羟甲基)-1,3-丙二醇,1,3-异苯并呋喃二酮和2,2-氧代二乙醇,2-丙烯酸酯
岩大戟内酯B
岩大戟内酯A
密叶辛木素