N-(9-菲基)乙酰胺 | 4235-09-0
中文名称
N-(9-菲基)乙酰胺
中文别名
——
英文名称
9-(N-acetylamino)phenanthrene
英文别名
N-(phenanthren-9-yl)acetamide;N-9-phenanthrenylacetamide;N-[9]phenanthryl-acetamide;N-[9]Phenanthryl-acetamid;N-Acetyl-phenanthrylamin-(9);9-Acetamino-phenanthren;9-Phenanthrylacetamide;N-phenanthren-9-ylacetamide
CAS
4235-09-0
化学式
C16H13NO
mdl
——
分子量
235.285
InChiKey
WFBZMYPAVBCTCU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:377.68°C (rough estimate)
-
密度:1.0711 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:18
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2924299090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Studies in the Cyanine Dye Series. VIII. Dyes Derived from 2-Methylphenanthro-[9,10]-thiazole摘要:DOI:10.1021/ja01280a017
-
作为产物:描述:参考文献:名称:Schmidt,J.; Strobel, Chemische Berichte, 1901, vol. 34, p. 1463摘要:DOI:
文献信息
-
Ruthenium-Catalyzed Hydrogenation of Carbocyclic Aromatic Amines: Access to Chiral Exocyclic Amines作者:Zhong Yan、Huan-Ping Xie、Hong-Qiang Shen、Yong-Gui ZhouDOI:10.1021/acs.orglett.7b04060日期:2018.2.16The first highly enantioselective hydrogenation of carbocyclic aromatic amines has been successfully realized using in situ-generated chiral ruthenium complex as catalyst, affording facile access to chiral exocyclic amines with up to 98% ee.使用原位生成的手性钌配合物作为催化剂,已经成功实现了碳环芳族胺的第一个高对映选择性加氢反应,可轻松获得ee高达98%的手性环外胺。
-
Fluorination of activated aromatic systems with Selectfluor™ F-TEDA-BF4 in ionic liquids作者:Mohammad Reza Poor HeraviDOI:10.1016/j.jfluchem.2007.11.006日期:2008.3Selectfluor™ was shown to be soluble in ionic liquid, thus allowing the ‘green’ electrophilic fluorination of activated aromatic systems compounds in high chemoselectivity and yields.
-
Aromatic nitro-compounds. Studies of the amination of 9-nitrophenanthrene and of the mononitrobiphenylenes作者:J. W. Barton、A. R. Grinham、K. E. WhitakerDOI:10.1039/j39710001384日期:——Nucleophilic aminations of 9-nitrophenanthrene and of 2-nitrobiphenylene have given 9-amino-10-nitrophenanthrene (I; R = NH2) and 2-amino-3-nitrobiphenylene (III; R = NH2) respectively; under the same conditions 1-nitrobiphenylene is unreactive. Some reactions of amines (I; R = NH2) and (III; R = NH2) are described.9-硝基菲和2-硝基联苯的亲核胺化分别得到9-氨基-10-硝基菲(I; R = NH 2)和2-氨基-3-硝基联苯(III; R = NH 2)。在相同条件下,1-硝基联苯是不反应的。描述了胺(I; R = NH 2)和(III; R = NH 2)的一些反应。
-
一种钌催化芳基胺化合物的不对称氢化合成 手性三级胺的方法
-
A Catalytic Method to Activate Nitromethane by the Cooperation of Homo‐ and Heterogeneous Catalysis作者:Weijin Wang、Jianzhong Liu、Licheng Yang、Song Song、Ning JiaoDOI:10.1002/anie.202312354日期:2024.2.12A catalytic activation of bulk chemical nitromethane (MeNO2) enables the facile synthesis of amides and nitriles from ketone, aldehyde, and alkyne substrates. This method eliminates the need for stoichiometric reductants or activators, opening up new possibilities for MeNO2 as a nitrogen source in organic synthesis.
表征谱图
-
氢谱1HNMR
-
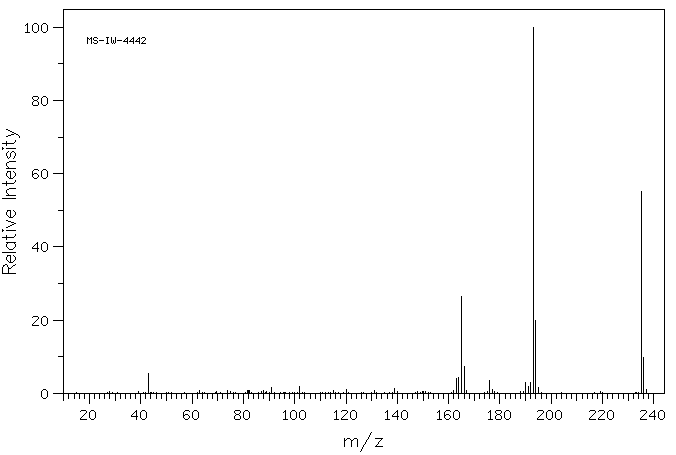
质谱MS
-
碳谱13CNMR
-
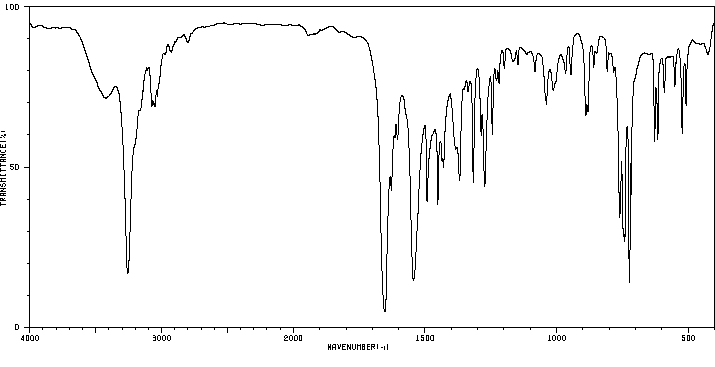
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩