N-2-氰乙基吡咯 | 43036-06-2
中文名称
N-2-氰乙基吡咯
中文别名
1-(2-氰基乙基)吡咯;N-(2-氰基乙基)吡咯;1-吡咯丙腈; 1-吡咯丙腈
英文名称
3-(pyrrol-1-yl)propanenitrile
英文别名
3-(1H-Pyrrol-1-yl)propanenitrile;3-pyrrol-1-ylpropanenitrile
CAS
43036-06-2
化学式
C7H8N2
mdl
MFCD00003092
分子量
120.154
InChiKey
IYOLJLGYJMJLSU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:132-133 °C/10 mmHg (lit.)
-
密度:1.048 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
溶解度:溶于氯仿
-
稳定性/保质期:
常规情况下不会分解,也没有危险的反应。
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:9
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:28.7
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S23
-
危险品运输编号:UN 2810
-
海关编码:2933990090
-
危险品标志:Xn
-
危险类别码:R20/21
-
WGK Germany:3
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302+H312+H332,H315,H319,H335
-
储存条件:密封、阴凉、干燥保存
SDS
| Name: | N-(2-Cyanoethyl)pyrrole 99+% Material Safety Data Sheet |
| Synonym: | 1-Pyrrolepropionitrile; 1-(2-Cyanoethyl)pyrrole; 3-(Pyrrol-1-yl)propiononitrile |
| CAS: | 43036-06-2 |
Synonym:1-Pyrrolepropionitrile; 1-(2-Cyanoethyl)pyrrole; 3-(Pyrrol-1-yl)propiononitrile
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 43036-06-2 | N-(2-Cyanoethyl)pyrrole | >99 | 256-051-6 |
Risk Phrases: 20/21
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation and in contact with skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Chronic exposure to cyanide solutions may lead to the development of a "cyanide" rash, characterized by itching, and by macular, papular, and vesicular eruptions, and may be accompanied by secondary infections. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Do NOT get water inside containers. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use dry chemical, carbon dioxide, alcohol-resistant foam, or water spray.
Cool containers with flooding quantities of water until well after fire is out.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 43036-06-2: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear yellow
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 132-133 deg C @ 10 mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 112 deg C (> 233.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.0480g/cm3
Molecular Formula: C7H8N2
Molecular Weight: 120.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen cyanide, carbon monoxide, oxides of nitrogen, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 43036-06-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
N-(2-Cyanoethyl)pyrrole - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: I
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: I
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: I
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21 Harmful by inhalation and in contact with
skin.
Safety Phrases:
S 23 Do not inhale gas/fumes/vapour/spray.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 43036-06-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 43036-06-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 43036-06-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1H-吡咯-1-丙胺 3-pyrrol-1-yl-propylamine 60794-90-3 C7H12N2 124.186 吡咯-1-丙酸 N-(2-carboxyethyl)pyrrole 89059-06-3 C7H9NO2 139.154
反应信息
-
作为反应物:参考文献:名称:Conductive microrod preparation by molecular self-assembly and polymerization摘要:通过蒸发诱导自组装(EISA)以及随后聚合带有吡咯端基的新型自组装分子,制备了导电微晶块。新合成的 N′1,N′6-双(3-(1-吡咯基)丙酰基)己二肼自组装分子从稀释溶液中自组装成微晶。吡咯环堆积是分子自组织成微晶的关键驱动力。自组装完成后,微晶表面的吡咯基团通过化学聚合使微晶具有导电性。聚合微晶的导电性与其他导电聚合物微晶相当。对聚合微晶的分析表明,聚合只发生在微晶组件的表面。这项研究证明了自组装和聚合生成复杂结构功能材料的概念,对设计功能性自组装分子具有重要价值。DOI:10.1039/c3ra40250a
-
作为产物:描述:3-(二烯丙基氨基)丙腈 在 RuCl2(1,3-dimesityl-imidazolidin-2-yl)(PCy3)(=CHPh) 、 ruthenium trichloride 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 12.0h, 以30%的产率得到N-2-氰乙基吡咯参考文献:名称:Pyrrole synthesis using a tandem Grubbs’ carbene–RuCl3 catalytic system摘要:A straightforward pyrrole synthesis from diallylamines is developed by using a tandem catalyst system leading to ring-closing metathesis with the second generation Grubbs' catalyst (10%) followed by dehydrogenation in the presence of RuCl3 x H2O (2%). (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2004.10.044
文献信息
-
二氢吡咯并五元杂芳基取代的氧杂螺环衍生物、其制法与医药上的用途
-
Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes作者:Indrajit Ghosh、Jagadish Khamrai、Aleksandr Savateev、Nikita Shlapakov、Markus Antonietti、Burkhard KönigDOI:10.1126/science.aaw3254日期:2019.7.26Two-for-one approach to photoredox In photoredox catalysis, an excited chromophore typically activates a single reactant either by oxidizing or reducing it. Ghosh et al. used a semiconductor catalyst to activate two reactants at once by quenching both an excited electron and the residual positive hole (see the Perspective by Swift). As such, two different reactive carbon or halide fragments could be光氧化还原二合一方法 在光氧化还原催化中,激发的发色团通常通过氧化或还原单个反应物来激活它。戈什等人。使用半导体催化剂通过淬灭激发的电子和残留的空穴来同时激活两种反应物(参见 Swift 的观点)。因此,可以将两个不同的反应性碳或卤化物片段附加到芳环上的不同位点。该催化剂还可以耐受氰化物等强亲核试剂,并且可以轻松回收和重复使用。科学,这个问题 p。360; 另见第。320 半导体光催化剂上氧化和还原位点的形成促进了双自由基加成反应。半导体表面上的光激发电子-空穴对可以与两种不同的基材进行氧化还原反应。与传统的电合成类似,主要的氧化还原中间体仅提供单独的氧化和还原产物,或者更罕见地结合成一种加成产物。在这里,我们报告了一种稳定的有机半导体材料,介孔石墨碳氮化物 (mpg-CN),可以充当可见光光氧化还原催化剂,以协调氧化和还原界面电子转移到两个或三个组件中的两种不同基材。用于芳烃和杂芳烃的直接双重碳氢功能化的系统。mpg-CN
-
Novel amine-catalysed hydroalkoxylation reactions of activated alkenes and alkynes作者:Julie E. Murtagh、S�amus H. McCooey、Stephen J. ConnonDOI:10.1039/b414895a日期:——Substoichiometric loadings of DBU catalyse the efficient 1,4-addition of alcohols and non-nucleophilic amines such as pyrrole to activated alkenes; the application of this methodology in a one-pot synthesis of a natural product, and as a novel strategy for the synthesis of mono-protected 1,3-carbonyl compounds is reported.
-
Functionalization of polypyrroles with acids and β-diketones as complexing groups. Part 1: electrochemical synthesis and properties作者:Ste´phane Roux、Pierre Audebert、Jacques Pagetti、Maxime RocheDOI:10.1039/b002460k日期:——The synthesis of pyrroles functionalized by complexing groups such as acetylacetone, dibenzoylmethane or carboxylic acids is described, as well as their electrooxidation into functionalized polypyrroles. We have studied the behavior of poly(functionalized pyrrole) films in the presence of Li+, Ni2+ and Co2+ cations by cyclic voltammetry (CV) and infrared (IR) spectroscopy. The electrochemical response under various conditions differs with the nature of the complexing group. We have also demonstrated the possibility to electrochemically generate copolymers with pyrrole and each functionalized pyrrole. The proportion of functional groups in the copolymers was estimated by following the peak potential dependence in cyclic voltammetry and was confirmed by IR spectroscopy.
-
C−H Alkenylation of Pyrroles by Electronically Matching Ligand Control作者:Hyun Tae Kim、Woohyeong Lee、Eunmin Kim、Jung Min JooDOI:10.1002/asia.201800558日期:2018.9.4Directing group and substrate control strategies have frequently been employed for the regioselective C−H alkenylation of acid‐ and oxidant‐sensitive pyrrole heterocycles. We developed an undirected, aerobic strategy for the C−H alkenylation of N‐alkylpyrroles by ligand control. For C2‐alkenylation of electron‐rich N‐alkylpyrroles, an electrophilic palladium catalyst derived from Pd(OAc)2 and 4,5‐diazafluoren‐9‐one
表征谱图
-
氢谱1HNMR
-
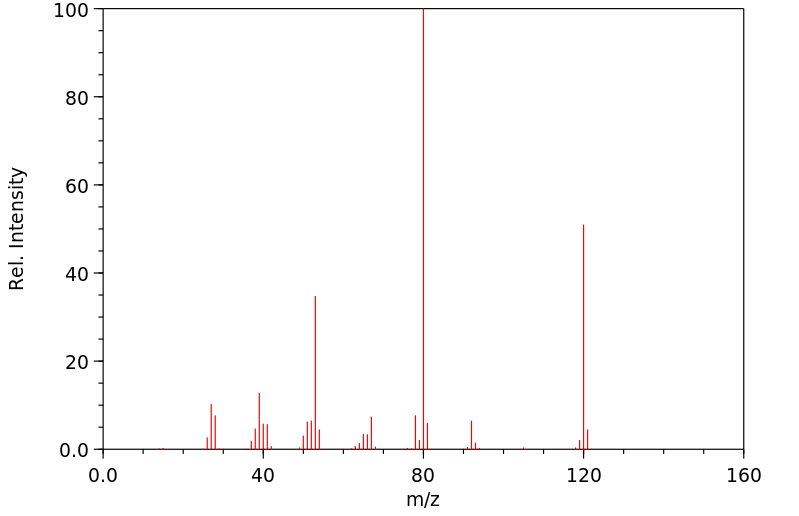
质谱MS
-
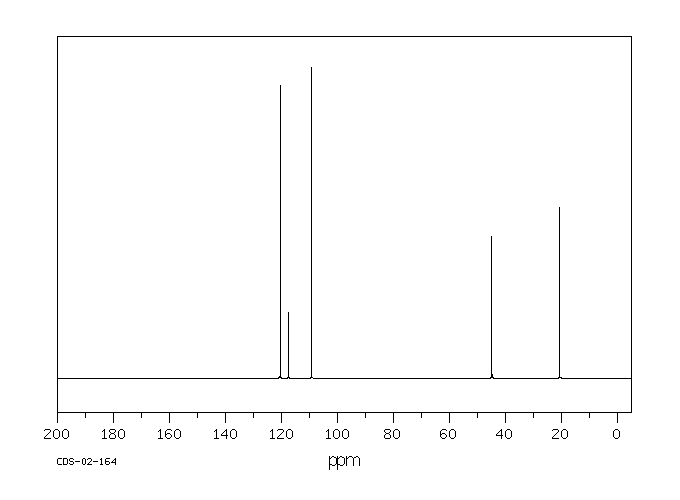
碳谱13CNMR
-
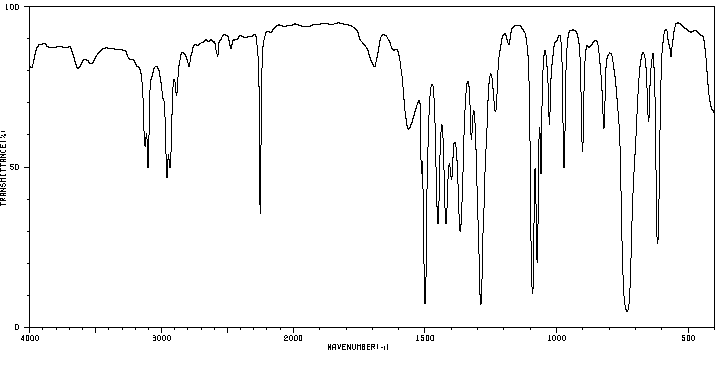
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄胆红酸
高树蛙毒素
颜料红2254
阿根诺卡菌素
阿托伐他汀镁
阿托伐他汀钙阿托伐他汀钙中间体1甲酯
阿托伐他汀钙杂质59
阿托伐他汀钙杂质52
阿托伐他汀钙杂质43
阿托伐他汀钙杂质
阿托伐他汀钙杂质
阿托伐他汀钙三水合物
阿托伐他汀钙L-8
阿托伐他汀钙
阿托伐他汀酸异丙酯
阿托伐他汀酰基-Β-D-葡糖苷酸
阿托伐他汀缩丙酮
阿托伐他汀相关化合物E
阿托伐他汀甲酯
阿托伐他汀甲胺盐
阿托伐他汀烯丙基酯
阿托伐他汀杂质F
阿托伐他汀杂质95
阿托伐他汀杂质5
阿托伐他汀杂质31
阿托伐他汀杂质1
阿托伐他汀叔丁酯
阿托伐他汀双氟杂质中间体
阿托伐他汀内酯-[D5]
阿托伐他汀内酯
阿托伐他汀乙酯
阿托伐他汀USP相关物质E
阿托伐他汀L1二胺物杂质
阿托伐他汀3-羟基消除杂质
阿托伐他汀3-氧杂质
阿托伐他汀
阿利考昔
阿伐他汀钠
镍(II)(吡唑二氰胺)2
镉原卟啉IX二甲酯
铬,二溴二(吡啶)-
达考帕泛
费耐力
角质形成细胞分化诱导剂
西拉美新盐酸盐
西拉美新
虫螨腈
萨格列扎
苏尼替尼N-1
芬度柳