N-己基丁酰胺 | 10264-17-2
中文名称
N-己基丁酰胺
中文别名
——
英文名称
N-hexyl-butanamide
英文别名
Butanamide, N-hexyl-;N-hexylbutanamide
CAS
10264-17-2
化学式
C10H21NO
mdl
——
分子量
171.283
InChiKey
YRVDTEDFGDNSLD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1434
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:12
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.9
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2924199090
SDS
反应信息
-
作为反应物:描述:N-己基丁酰胺 在 Iron(III) nitrate nonahydrate 、 sodium bromate 、 氧气 作用下, 以 乙腈 为溶剂, 以42 %的产率得到N-butyrylhexanamide参考文献:名称:通过可见光驱动的铁催化选择性 α-氧化酰胺摘要:羟基自由基(˙OH)作为一种高活性物质,可以与多种化学物质发生无选择性的反应。 ˙OH自由基通常在恶劣条件下产生。在此,我们报道了在 30 W 功率照射下,通过Fe(NO 3 ) 3 ·9H 2 O 催化剂内球途径,在更绿色和温和的条件下,羟基自由基诱导的酰胺选择性N -α C(sp 3 )–H 键氧化使用NaBrO 3作为氧化剂的蓝色LED灯带( λ = 455 nm)。该方案表现出高化学选择性和优异的官能团耐受性。初步的机理研究表明,铁催化剂通过铁配合物的可见光诱导均裂(VLIH)和随后的氢原子转移(HAT)过程提供羟基自由基,以实现这种转变。DOI:10.1039/d3ob01984e
-
作为产物:参考文献:名称:三苯基锑二羧酸盐的氨解及其在催化酰胺化中的应用摘要:三苯基锑二羧酸盐(Ph3Sb(O2CR)2,其中 R=Me、CF3、Ph 和 CH2NH-Z)容易与胺(R'NH2,其中 R'=n-C6H13、s-Bu、C6H11 和 Ph)反应得到相应的酰胺和三苯基氧化锑,收率相当好。RCO2H与R'NH2的酰胺化也由有机锑化合物催化。DOI:10.1246/cl.1986.1901
文献信息
-
Metal-free, hydroacylation of CC and NN bonds via aerobic C–H activation of aldehydes, and reaction of the products thereof作者:Vijay Chudasama、Ahmed R. Akhbar、Karim A. Bahou、Richard J. Fitzmaurice、Stephen CaddickDOI:10.1039/c3ob41632a日期:——In this report, a thorough evaluation of the use of aerobically initiated, metal-free hydroacylation of various CC and NN acceptor molecules with a wide range of aldehydes is presented. The aerobic-activation conditions that have been developed are in sharp contrast to previous conditions for hydroacylation, which tend to use transition metals, peroxides that require thermal or photochemical degradation, or N-heterocyclic carbenes. The mildness of the conditions enables a number of reactions involving sensitive reaction partners and, perhaps most significantly, allows for α-functionalised chiral aldehydes to undergo radical-based hydroacylation with complete retention of optical purity. We also demonstrate how the resulting hydroacylation products can be transformed into other useful intermediates, such as γ-keto-sulfonamides, sultams, sultones, cyclic N-sulfonyl imines and amides.
-
Functionalisation of aldehydes via aerobic hydroacylation of azodicarboxylates ‘on’ water作者:Vijay Chudasama、Jenna M. Ahern、Dipti V. Dhokia、Richard J. Fitzmaurice、Stephen CaddickDOI:10.1039/c0cc04520a日期:——Herein we report the functionalisation of aldehydesvia hydroacylation of azodicarboxylates. A range of functionalised aldehydes are employed as the limiting reagent including chiral non-racemic aldehydes bearing α-stereocentres which are functionalised giving access to enantiomerically pure products. The resultant hydrazides can be employed as acyl donors in the synthesis of amides.
-
Rapid Vortex Fluidics: Continuous Flow Synthesis of Amides and Local Anesthetic Lidocaine作者:Joshua Britton、Justin M. Chalker、Colin L. RastonDOI:10.1002/chem.201501785日期:2015.7.20on multigram scales. Amide synthesis under flow was also extended to a total synthesis of local anesthetic lidocaine, with sequential reactions carried out in two serially linked VFD units. The synthesis could also be executed in a single VFD, in which the tandem reactions involve reagent delivery at different positions along the rapidly rotating tube with in situ solvent replacement, as a molecular
-
A Novel Dehydrazination Reaction. V. The Formation of Various Amides from Aliphatic and Aromatic Carboxylic Acid Hydrazides in the Presence of Chloral作者:Tetsuji Kametani、Osamu UmezawaDOI:10.1248/cpb.14.369日期:——In the previous papers the reactions between aromatic, aliphatic and heterocyclic carboxylic acid hydrazides and either chloral or bromal in various alcohols were attempted and respective esters were obtained. In this paper the reactions of aromatic and aliphatic acid hydrazide with chloral in the presence of various amines were examined, leading eventually to reveal the formation of our expected acid amides as are shown in Table I and II. The intermediates in this reaction, 1-benzoyl-2-(2, 2, 2-trichloroethylidene) hydrazine (III : R=C6H5-, X=Cl) was found to form the amides (VI) when heated in amines. This fact indicated that the acid hydrazides converted to their amides through III.
-
On the Thermal Dissociation of Organic Compounds. VI. The Effect of the Substituent and That of the Solvent on the Thermal Dissociation of Ureas作者:Teruaki Mukaiyama、Sh\={o}ichiro Ozaki、Toshio HoshinoDOI:10.1246/bcsj.27.578日期:1954.9rate constants of the thermal dissociation of 1,1-dimethyl, 1,3-diethyl, 1,3-diisopropyl, 1,1, 3-triethyl, 1, 3-dihexyl, 1,3-dibenzyl, 1, 1-diethyl-3-isopropyl and 1,3-di-t-butyl ureas in n-butyric,n-caproic,n-capric, phenylacetic and benzoic acids were deter mined. The effects of substituents and those of solvents on the reaction rate were discussed. The transition state of the dissociation containing
表征谱图
-
氢谱1HNMR
-
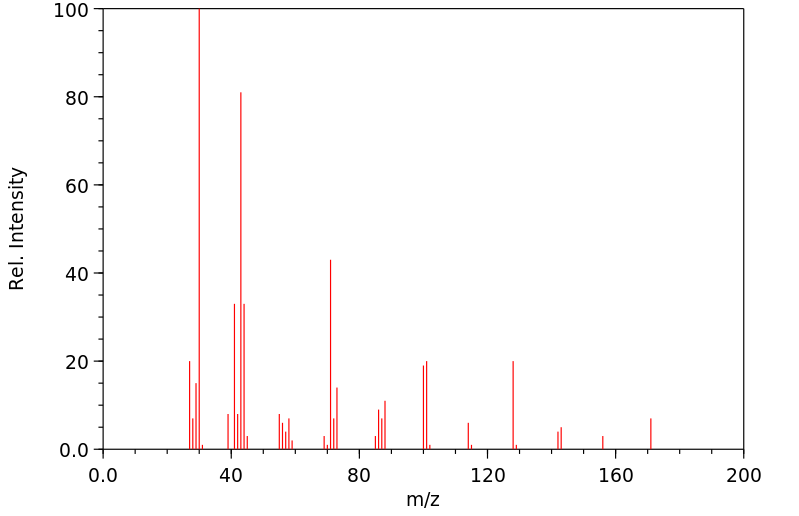
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯