硫丹 | 115-29-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:106°C
-
沸点:106 °C(Press: 0.70 Torr)
-
密度:1.7450
-
闪点:-26 °C
-
溶解度:可溶于氯仿(少量)、DMSO(少量)、甲醇(少量)
-
物理描述:Beta - endosulfan appears as brown crystals. Melting point 208-210°C. Used as an insecticide.
-
颜色/状态:Brown crystals ... [Note: Technical product is a tan, waxy, isomer mixture].
-
气味:Similar to terpene.
-
蒸汽压力:1.73X10-7 at 25 °C
-
亨利常数:2.42e-05 atm-m3/mole
-
稳定性/保质期:
-
分解:When heated to decomposition it emits toxic fumes of /hydrogen chloride and sulfoxides/.
-
腐蚀性:Corrosive to iron
-
保留指数:2177;2192
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:19
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.777
-
拓扑面积:54.7
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
职业暴露等级:D
-
职业暴露限值:TWA: 0.1 mg/m3 [skin]
-
危险等级:6.1(a)
-
危险品标志:T,N,Xn,F,T+
-
安全说明:S28,S36/37,S45,S60,S61,S62
-
危险类别码:R11,R67,R24/25,R51/53,R48/20,R65,R62,R36,R38,R50/53
-
WGK Germany:3
-
海关编码:29209090
-
包装等级:II
-
危险类别:6.1(a)
制备方法与用途
毒性 大鼠急性经口LD50为40~50mg/kg(原药22.7~160mg/kg,雄),22.7mg/kg(雌)。大鼠急性经皮LD50>500mg/kg,兔359mg/kg。大鼠吸入LC50为34.5mg/L (雄,4h)、12.6mg/L (雌,4h)。对皮肤和眼睛有轻度刺激,无致敏性。大鼠13周喂养试验的无作用剂量为10mg/kg饲料或0.7mg/kg体重。大鼠2年喂养试验的无作用剂量为15mg/kg饲料或0.6~0.7mg/kg体重。致突变试验阴性,大鼠2代繁殖未见不良影响。试验条件下未见致癌作用。母鸡试验未见迟发性神经毒性。联合国粮农组织和世界卫生组织联席会议推荐的ADI为0.006mg/kg体重(1989年)。药剂对鱼类高毒,LC50约0.002mg/L(96h),野鸭急性经口LD50为200~750mg/kg,野鸡620~1000mg/kg,鹌鹑85~106mg/kg。蜜蜂接触LD50为7.1μg/只,经口LD50为6.9μg/只。
化学性质 茶色或白色晶体
用途 有机氯杀虫剂。具有触杀和胃毒作用,杀虫谱广,持效期长。气温高于20℃时,也可通过其蒸气起杀虫作用。可用于棉花、果树、大豆、茶、蔬菜、烟草、马铃薯及苜蓿等作物,防治对昆虫具有触杀及胃毒作用的害虫。常用方法为用20%乳油300~500倍液喷雾来防治棉铃虫、红铃虫、棉卷叶蛾、金刚钻、金龟子、梨小食心虫、桃小食心虫、黏虫、蓟马、叶蝉、蚜虫、菜青虫、天牛等害虫。防治棉花和果树上的蚜虫、螨则用20%乳油500~1000倍液喷雾,用20%乳油200倍液灌根可防治地老虎。近年来,硫丹用于防治抗性棉铃虫效果良好,尤其与氯氰菊酯混用时更受欢迎。食用或饲料作物收获前3周停用。
生产方法 硫丹由六氯环戊二烯与1,4-丁烯二醇加热反应,保温10h后冷却、过滤、洗涤制得硫丹醇,再将硫丹醇和溶剂加热到一定温度,滴加氯化亚砜,维持反应1h,减压脱氯化氢,冷却、结晶过滤制得硫丹。详见参考文献[3]。
类别 农药
毒性分级 剧毒
可燃性危险特性 受热放出有毒氯化物和氧化硫气体;遇酸、碱或潮气分解产生有毒氧化硫气体
储运特性 库房应通风低温干燥,与食品原料分开储运
灭火剂 砂土、干粉、泡沫
职业标准 纯品 TWA 0.1 毫克/立方米; STEL 0.3 毫克/立方米
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— General Weed Killer —— C9H6Cl6O3S 406.9 硫丹硫酸酯 endosulfan sulfate 1031-07-8 C9H6Cl6O4S 422.928 硫丹醇 1,2,3,4,7,7-hexachloro-5,6-bis(hydroxymethyl)bicyclo<2.2.1>hept-2-ene 2157-19-9 C9H8Cl6O2 360.879 硫丹乙酯 endosulfan ether 3369-52-6 C9H6Cl6O 342.864 硫丹内酯 endosulfan lactone 3868-61-9 C9H4Cl6O2 356.848
反应信息
-
作为反应物:描述:参考文献:名称:NAQVI, SYED M.;NEWTON, DEBORAH J., J. ENVIRON. SCI. AND HEALTH. B, 25,(1990) N, C. 511-526摘要:DOI:
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
Thieno-pyrimidine compounds having fungicidal activity
表征谱图
-
氢谱1HNMR
-
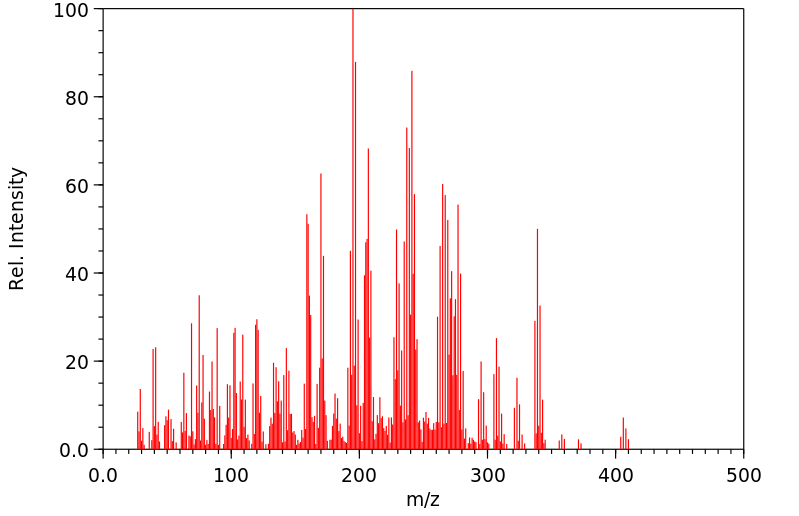
质谱MS
-
碳谱13CNMR
-
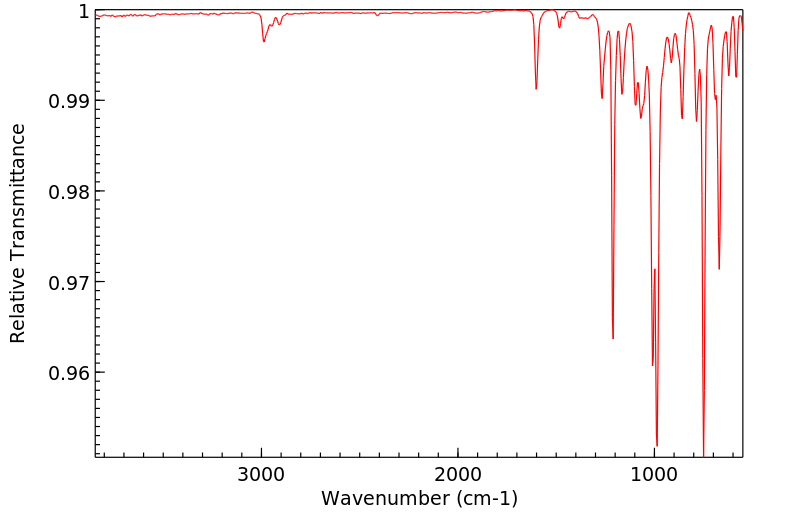
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息