2,6-二甲基-5-碘-4(3H-)-嘧啶酮 | 83410-37-1
中文名称
2,6-二甲基-5-碘-4(3H-)-嘧啶酮
中文别名
——
英文名称
5-iodo-2,6-dimethylpyrimidin-4-ol
英文别名
5-Iodo-2,6-dimethylpyrimidin-4-ol;5-iodo-2,4-dimethyl-1H-pyrimidin-6-one
CAS
83410-37-1
化学式
C6H7IN2O
mdl
——
分子量
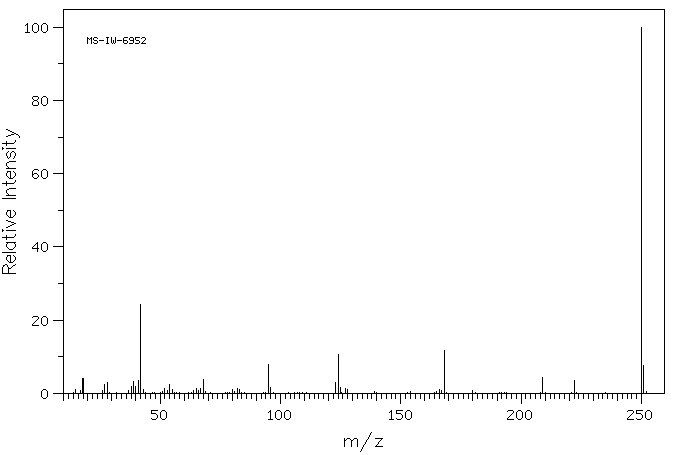
250.039
InChiKey
LLRCHCURQABDLS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:196-199 °C
-
沸点:254.9±43.0 °C(Predicted)
-
密度:2.03±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:41.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2933599090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二甲基-6-羟基嘧啶 4-hydroxy 2,6-dimethylpyrimidine 6622-92-0 C6H8N2O 124.142 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-iodo-3-(methoxymethyl)-2,6-dimethylpyrimidin-4(3H)-one 1369769-10-7 C8H11IN2O2 294.092
反应信息
-
作为反应物:描述:2,6-二甲基-5-碘-4(3H-)-嘧啶酮 在 四(三苯基膦)钯 、 碳酸氢钠 、 lithium chloride 、 三氯氧磷 作用下, 以 1,4-二氧六环 、 甲苯 、 正丁醇 为溶剂, 反应 96.0h, 生成 4-(4-bromobenzylamino)-2,6-dimethyl-5-(1-ethoxyvinyl)pyrimidine参考文献:名称:Metabolites of the Angiotensin II Antagonist Tasosartan: The Importance of a Second Acidic Group摘要:Described in this paper is the synthesis and pharmacological activity of five metabolites of the angiotensin II antagonist tasosartan (1). Of particular interest is the effect of the additional acidic group of the enol metabolite (8) on activity. As suggested by the structural-activity relationship of other angiotensin II antagonist series, a second acidic group can improve receptor binding activity but decrease in vivo activity after oral dosing. The metabolic introduction of a second acidic group in tasosartan bypasses this problem and contributes to the excellent profile of the compound. A molecular modeling study provides a rationale for the role of the enol group of 8 in AT(1) receptor binding.DOI:10.1021/jm970690q
-
作为产物:描述:参考文献:名称:通过钯催化炔基嘧啶与芳基碘化物的环化反应,合成新型5,6-取代的呋喃并[2,3- d ]嘧啶摘要:描述了一种合成5,6-二取代的呋喃并[2,3- d ]嘧啶衍生物的灵活方法。关键步骤是用各种芳基碘化物对炔基嘧啶进行钯催化的芳基环化反应,标题化合物的收率为36-75%。DOI:10.1016/j.tet.2006.12.077
文献信息
-
Compounds for treating spinal muscular atrophy申请人:PTC Therapeutics Inc.公开号:US09617268B2公开(公告)日:2017-04-11Provided herein are compounds, compositions thereof and uses therewith for treating spinal muscular atrophy. In a specific embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN2 into mRNA that is transcribed from the SMN2 gene. In another specific embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN1 into mRNA that is transcribed from the SMN1 gene. In yet another embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN1 and SMN2 into mRNA that is transcribed from the SMN1 and SMN2 genes, respectively.
-
CYCLOPROPANE COMPOUND申请人:Terauchi Taro公开号:US20120095031A1公开(公告)日:2012-04-19A cyclopropane compound represented by the following formula (A) or a pharmaceutically acceptable salt thereof has orexin receptor antagonism, and therefore has a potencial of usefulness for the treatment of sleep disorder for which orexin receptor antagonism is effective, for example, insomnia: wherein Q represents —CH— or a nitrogen atom, R 1a and R 1b each independently represent a C 1-6 alkyl group and the like, R 1c represents a hydrogen atom and the like, R 2a , R 2b , R 2c and R 2d each independently represent a hydrogen atom, a halogen atom, a C 1-6 alkyl group and the like, R 3a , R 3b and R 3c each independently represent a hydrogen atom, a halogen atom and the like, and R 3d represents a hydrogen atom and the like.
-
Method for synthesis of AZA-annelated pyrroles, thiophenes, and furans
-
[EN] EZH2 INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS D'EZH2 ET LEURS UTILISATIONS申请人:DANA FARBER CANCER INST INC公开号:WO2016073956A1公开(公告)日:2016-05-12The present disclosure provides compounds of any one of Formulae (I) and (II). The compounds described herein are inhibitors of histone methyltransferases (e.g., enhancer of zeste homolog 1 (EZH1) and enhancer of zeste homolog 2 (EZH2)) and are useful in treating and/or preventing a broad range of diseases (e.g., proliferative diseases). Also provided in the present disclosure are pharmaceutical compositions, kits, methods, and uses including or using a compound described herein. Further provided in the present disclosure are methods of identifying EZH1and/or EZH2 inhibitors.
-
Studies on pyrimidine derivatives. XXVIII. Synthesis of pyridopyrimidine derivatives by cross-coupling of halopyrimidines with olefins and acetylenes.作者:TAKAO SAKAMOTO、YOSHINORI KONDO、HIROSHI YAMANAKADOI:10.1248/cpb.30.2410日期:——Three kinds of pyridopyrimidines were synthesized from appropriate pyrimidine derivatives. Cross-coupling of 4-amino-5-iodopyrimidines with α, β-unsaturated carboxylic esters followed by ring-closure afforded pyrido [2, 3-d] pyrimidines. Ammonolysis of 4-ethoxycarbonyl-5-phenylethynyl-and 5-ethoxycarbonyl-4-phenyl-ethynyl-pyrimidines which were obtained by cross-coupling of the corresponding halopyrimidines with phenylacetylene, gave pyrido [3, 4-d]-and pyrido [4, 3-d]-pyrimidines, respectively.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
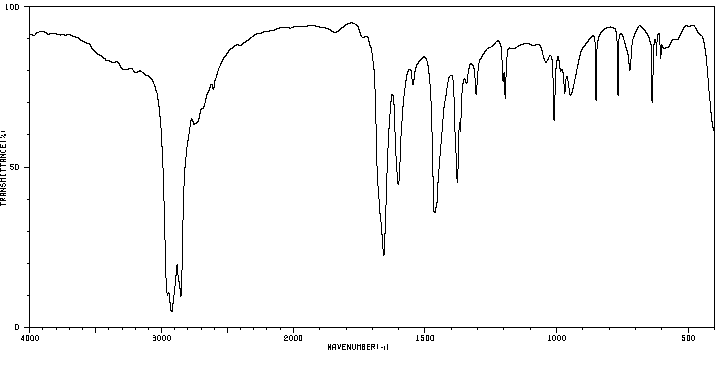
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3