1-(2-氯苯基)环丙烷羧酸 | 122143-19-5
中文名称
1-(2-氯苯基)环丙烷羧酸
中文别名
1-(2-氯苯基)环丙甲酸;1-(2-氯苯基)环丙基甲酸;1-(2-氯-苯基)-环丙烷甲酸
英文名称
1-(2-chlorophenyl)cyclopropanecarboxylic acid
英文别名
1-(2-chlorophenyl)cyclopropane-1-carboxylic acid
CAS
122143-19-5
化学式
C10H9ClO2
mdl
MFCD07374435
分子量
196.633
InChiKey
CODFVANRHMDSSS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:159-162 °C
-
沸点:345.9±35.0 °C(Predicted)
-
密度:1.403±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:13
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2916399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1-(2-Chlorophenyl)cyclopropanecarboxylic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-(2-Chlorophenyl)cyclopropanecarboxylic acid
CAS number: 122143-19-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H9ClO2
Molecular weight: 196.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1-(2-Chlorophenyl)cyclopropanecarboxylic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-(2-Chlorophenyl)cyclopropanecarboxylic acid
CAS number: 122143-19-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H9ClO2
Molecular weight: 196.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 1-(2-chlorophenyl)cyclopropanecarboxylate 943118-88-5 C11H11ClO2 210.66 1-(2-氯苯基)环丙烷甲腈 1-(2-chlorophenyl)cyclopropane-1-carbonitrile 122143-18-4 C10H8ClN 177.633 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(2-氯苯基)环丙烷-1-甲酰氯 1-[1-(2-Chlorophenyl)cyclopropyl]carbonyl chloride 151154-92-6 C10H8Cl2O 215.079
反应信息
-
作为反应物:参考文献:名称:蛋白质-配体相互作用中卤素键的系统研究摘要:卤素键触发活性:通过将芳环H原子与Cl,Br和I交换,可以观察到一系列共价的人组织蛋白酶L抑制剂的结合亲和力增加,Cl,Br和I与S3口袋中的Gly61的CO基团进行卤素键合酶。相反,氟强烈避免卤素键(参见方案)。卤素键的强距离和角度依赖性已被证实适用于生物系统。DOI:10.1002/anie.201006781
-
作为产物:描述:methyl 1-(2-chlorophenyl)cyclopropanecarboxylate 在 水 、 lithium hydroxide 、 sodium hydroxide 、 盐酸 作用下, 以 四氢呋喃 、 水 为溶剂, 反应 70.5h, 生成 1-(2-氯苯基)环丙烷羧酸参考文献:名称:蛋白质-配体相互作用中卤素键的系统研究摘要:卤素键触发活性:通过将芳环H原子与Cl,Br和I交换,可以观察到一系列共价的人组织蛋白酶L抑制剂的结合亲和力增加,Cl,Br和I与S3口袋中的Gly61的CO基团进行卤素键合酶。相反,氟强烈避免卤素键(参见方案)。卤素键的强距离和角度依赖性已被证实适用于生物系统。DOI:10.1002/anie.201006781
文献信息
-
[EN] INDANE DERIVATIVES AS MGLUR7 MODULATORS<br/>[FR] DÉRIVÉS D'INDANE UTILISÉS COMME MODULATEURS DE MGLUR7申请人:TAKEDA PHARMACEUTICALS CO公开号:WO2017131221A1公开(公告)日:2017-08-03The present invention provides compounds of formula (I) and pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4a and R4b are as defined in the specification, processes for their preparation, pharmaceutical compositions containing them and their use in therapy. The compounds of formula (I) are mGluR7 modulators.本发明提供了式(I)的化合物及其药学上可接受的盐,其中R1、R2、R3、R4a和R4b如规范中定义,其制备方法,含有它们的药物组合物以及它们在治疗中的用途。式(I)的化合物是mGluR7调节剂。
-
Methods and Compositions for Selectin Inhibition申请人:Kaila Neelu公开号:US20080255192A1公开(公告)日:2008-10-16The present teachings relate to novel compounds of formula I: wherein the constituent variables are as defined herein. Compounds of the present teachings can act as antagonists of the mammalian adhesion proteins known as selecting. Methods for treating or preventing selectin-mediated disorders are provided, which include administration of these compounds in a therapeutically effective amount.本教学涉及到式I的新化合物: 其中组成变量如本文所定义。本教学的化合物可以作为被称为选择素的哺乳动物粘附蛋白的拮抗剂。提供了治疗或预防选择素介导的疾病的方法,包括以治疗有效剂量给予这些化合物。
-
Discovery and Optimization of Pyrazole Amides as Inhibitors of ELOVL1作者:Jon H. Come、Timothy J. Senter、Michael P. Clark、John J. Court、Zachary Gale-Day、Wenxin Gu、Elaine Krueger、Jianglin Liang、Mark Morris、Suganthini Nanthakumar、Hardwin O’Dowd、Francois Maltais、Ganesh Iyer、John Andreassi、Christina Boucher、Tony Considine、Cameron S. Moody、William Taylor、Arun K. Mohanty、Yulin Huang、Harmon Zuccola、Joyce Coll、Kenneth C. Bonanno、Kevin J. Gagnon、Lu Gan、Fan Lu、Hong Gao、Ananthisrinivas Chakilam、Juntyma Engtrakul、Bin Song、Dan Crawford、Elisabeth Doyle、Tal Kramer、Bryan Vought、Jonathan Phillips、Raymond Kemper、Martin Sanders、Rebecca Swett、Brinley Furey、Ray Winquist、Mark E. Bunnage、Katrina L. Jackson、Paul S. Charifson、Sanjay S. MagaviDOI:10.1021/acs.jmedchem.1c00944日期:2021.12.23Accumulation of very long chain fatty acids (VLCFAs) due to defects in ATP binding cassette protein D1 (ABCD1) is thought to underlie the pathologies observed in adrenoleukodystrophy (ALD). Pursuing a substrate reduction approach based on the inhibition of elongation of very long chain fatty acid 1 enzyme (ELOVL1), we explored a series of thiazole amides that evolved into compound 27─a highly potent由于 ATP 结合盒蛋白 D1 (ABCD1) 的缺陷导致超长链脂肪酸 (VLCFA) 的积累被认为是在肾上腺脑白质营养不良 (ALD) 中观察到的病理学的基础。追求基于抑制超长链脂肪酸 1 酶 (ELOVL1) 延伸的底物还原方法,我们探索了一系列噻唑酰胺,它们演变成化合物27——一种高效的中枢神经系统 (CNS) 渗透化合物,具有有利的体内药代动力学。化合物27选择性抑制 ELOVL1,减少 ALD 患者成纤维细胞、淋巴细胞和小胶质细胞中 C26:0 VLCFA 的合成。在 ALD 小鼠模型中,化合物27治疗将血液中的 C26:0 VLCFA 浓度降低到接近野生型水平,在与疾病相关的组织大脑中降低高达 65%。皮肤、眼睛和中枢神经系统的临床前安全性发现阻止了进展;这些发现的起源和相关性需要进一步研究。ELOVL1 抑制是使 ALD 模型中 VLCFA 正常化的有效方法。
-
[EN] SUBSTITUTED HETEROCYCLIC DERIVATIVES<br/>[FR] DÉRIVÉS HÉTÉROCYCLIQUES SUBSTITUÉS申请人:HOFFMANN LA ROCHE公开号:WO2014079850A1公开(公告)日:2014-05-30The present invention relates to compounds of general formula (I-1) or (I-2) wherein R1 is hydrogen, lower alkyl, lower alkoxy, halogen, O(CH2)2-lower akoxy, O(CH2)2N(CH3)2, or O(CH2)-morpholinyl; R1' is hydrogen, lower alkyl, lower alkoxy, halogen, O(CH2)2-lower akoxy, O(CH2)2N(CH3)2, or O(CH2)-morpholinyl; with the proviso that both R1 and R1' may be simultaneously hydrogen, but only one of R1 and R1' is lower alkyl, lower alkoxy, halogen, O(CH2)2-lower akoxy, O(CH2)2N(CH3)2, or O(CH2)-morpholinyl; Het is a 5-or 6 membered heteroaryl group, wherein the heteroatom is selected from N, O or S; X is -CRR'-, -CRR'-NR'-, -C(O)-, -CH2-S-, -CH2-S(O)2-, CH2-O- or -CH2-CRR'-; R/R' are independently from each other hydrogen, lower alkyl, hydroxy or phenyl, or R and R' may form together with the carbon atom to which they are attached a cyclopropyl ring; R2 is lower alkyl, -C(O)O-lower alkyl, C3-6-cycloalkyl optionally substituted by lower alkyl or =O, bridged cyclohexyl or C3-6-cycloalkenyl, or is a 5-membered heteroaryl group, wherein the heteroatom is selected from N, O or S and which is optionally substituted by one or more lower alkyl, or is pyridinyl, optionally substituted by halogen or lower alkoxy; or is phenyl, optionally substituted by one or more R2', selected from halogen, cyano, S(O)2-lower alkyl, lower alkyl, lower alkyl substituted by halogen, lower alkoxy, lower alkoxy substituted by halogen or amino, or is benzo[1,3]dioxolyl, naphthyl, indolyl, benzo-isoxazolyl, 2,3-dihydro-1H-indenyl, optionally substituted by lower alkoxy or by an oxo group, or is 3,4-dihydro-2H- [1,4]oxazinyl, optionally substituted by an oxo group, or is a five or six membered heterocycloalkyl group; or to a pharmaceutically acceptable acid addition salt, to a racemic mixture or to its corresponding enantiomer and/or optical isomers thereof. The compounds may be used for the treatment or prophylaxis of schizophrenia, obsessive-compulsive personality disorder, major depression, bipolar disorders, anxiety disorders, normal aging, epilepsy, retinal degeneration, traumatic brain injury, spinal cord injury, post-traumatic stress disorder, panic disorder, Parkinson's disease, dementia, Alzheimer's disease, mild cognitive impairment, chemotherapy-induced cognitive dysfunction, Down syndrome, autism spectrum disorders, hearing loss, tinnitus, spinocerebellar ataxia, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, stroke, radiation therapy, chronic stress, abuse of neuro-active drugs, such as alcohol, opiates, methamphetamine, phencyclidine and cocaine.本发明涉及通式(I-1)或(I-2)的化合物,其中R1为氢、较低烷基、较低烷氧基、卤素、O(CH2)2-较低烷氧基、O( )2N(CH3)2或O( )-吗啡啉基;R1'为氢、较低烷基、较低烷氧基、卤素、O( )2-较低烷氧基、O( )2N( )2或O( )-吗啡啉基;但要注意,R1和R1'中两者同时为氢,但只有一个为较低烷基、较低烷氧基、卤素、O( )2-较低烷氧基、O( )2N( )2或O( )-吗啡啉基;Het为5或6元杂芳基团,其中杂原子选自N、O或S;X为-CRR'-、-CRR'-NR'-、-C(O)-、- -S-、- -S(O)2-、 -O-或- -CRR'-;R/R'独立地为氢、较低烷基、羟基或苯基,或R和R'可与它们连接的碳原子共同形成环丙基环;R2为较低烷基、-C(O)O-较低烷基、C3-6-环烷基(可选择地被较低烷基或=O取代)、桥环己基或C3-6-环烯基,或为5元杂芳基团,其中杂原子选自N、O或S,可选择地被一个或多个较低烷基取代,或为吡啶基,可选择地被卤素或较低烷氧基取代;或为苯基,可选择地被一个或多个来自卤素、氰基、S(O)2-较低烷基、较低烷基、被卤素取代的较低烷基、较低烷氧基、被卤素取代的较低烷氧基或氨基的R2'取代基,或为苯并[1,3]二氧杂环己基、萘基、吲哚基、苯并异噁唑基、2,3-二氢-1H-茚基,可选择地被较低烷氧基或氧羟基取代,或为3,4-二氢-2H-[1,4]噁唑基,可选择地被氧羟基取代,或为五元或六元杂环烷基团;或为药学上可接受的酸盐,或为外消旋混合物,或为其相应的对映体和/或光学异构体。这些化合物可用于治疗或预防精神分裂症、强迫性人格障碍、重性抑郁症、双相障碍、焦虑障碍、正常衰老、癫痫、视网膜退化、创伤性脑损伤、脊髓损伤、创伤后应激障碍、恐慌障碍、帕金森病、痴呆症、阿尔茨海默病、轻度认知障碍、化疗诱导的认知功能障碍、唐氏综合征、自闭症谱系障碍、听力损失、耳鸣、脊髓小脑性共济失调、肌萎缩侧索硬化、多发性硬化、亨廷顿病、中风、放射治疗、慢性压力、滥用神经活性药物,如酒精、阿片类药物、甲基苯丙胺、芬太尼和可卡因。
-
[EN] 5-ARYL-3,9-DIAZASPIRO[5.5]UNDECAN-2-ONE COMPOUNDS<br/>[FR] COMPOSÉS 5-ARYL-3,9-DIAZASPIRO[5.5]UNDÉCAN-2-ONE申请人:BAYER AG公开号:WO2020048830A1公开(公告)日:2020-03-12The present invention covers 5-aryl-3,9-diazaspiro[5.5]undecan-2-one compounds of general formula (I) and general formula (l-a): in which A, R1 and R2 are as defined herein, pharmaceutical compositions and combinations comprising said compounds and the use of said compounds for manufacturing pharmaceutical compositions for the treatment and/or prophylaxis of diseases, and compounds of general formula (l-c) and general formula (l-d): in which R1 and R2 are as defined herein, for the treatment and/or prophylaxis of diseases, pharmaceutical compositions and combinations comprising said compounds and the use of said compounds for manufacturing pharmaceutical compositions.本发明涵盖了一般式(I)和一般式(l-a)的5-芳基-3,9-二氮杂螺[5.5]十一碳酮化合物:其中A,R1和R2如本文所定义,含有所述化合物的药物组合物和组合物,以及利用所述化合物制造用于治疗和/或预防疾病的药物组合物的用途,以及一般式(l-c)和一般式(l-d)的化合物:其中R1和R2如本文所定义,用于治疗和/或预防疾病,含有所述化合物的药物组合物和组合物,以及利用所述化合物制造药物组合物的用途。
表征谱图
-
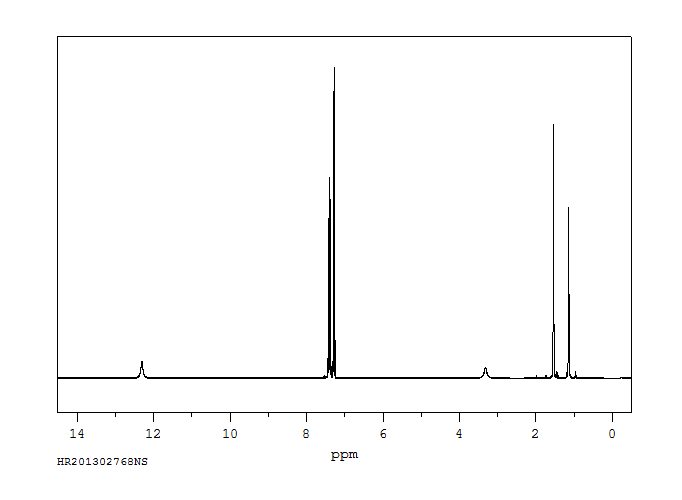
氢谱1HNMR
-
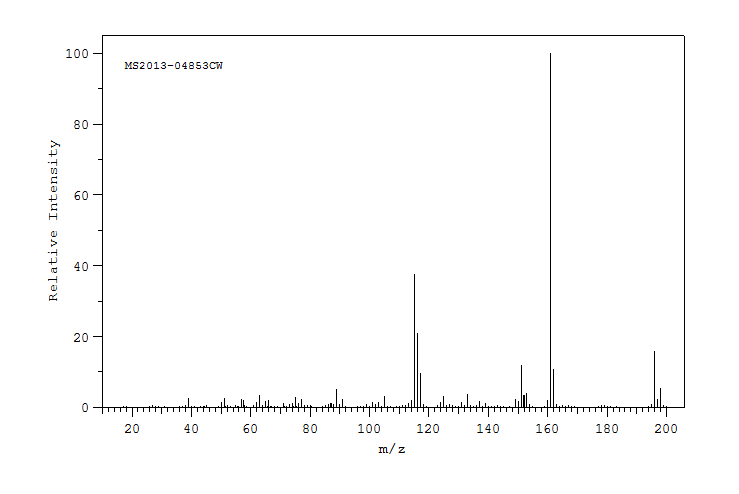
质谱MS
-
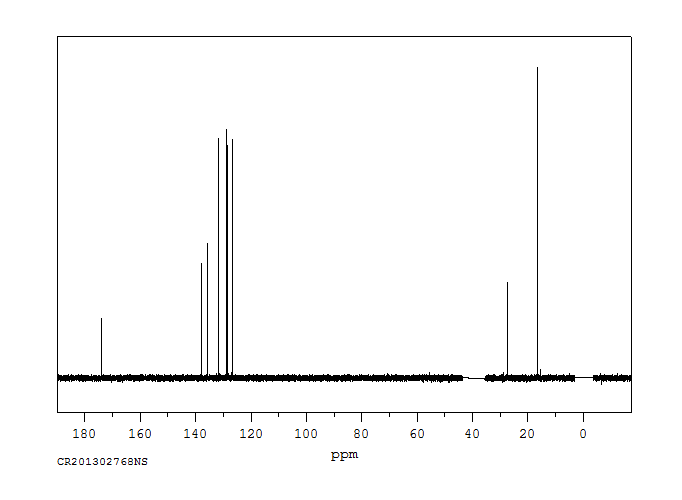
碳谱13CNMR
-
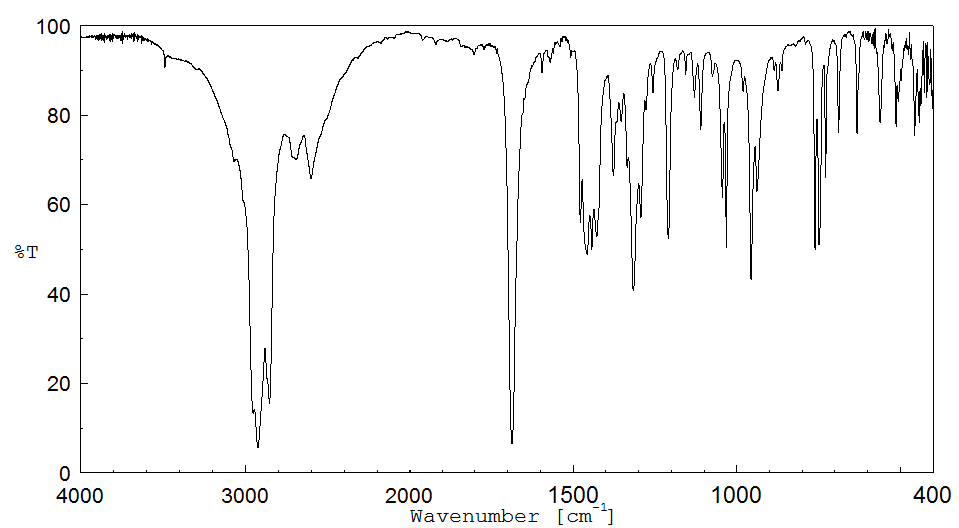
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫