2-氯-1,4-二乙氧基苯 | 52196-74-4
中文名称
2-氯-1,4-二乙氧基苯
中文别名
1-氯-2,5-二乙氧基苯;2,5-二乙氧基氯苯; 2,5-二乙氧基氯苯
英文名称
2-chloro-1,4-diethoxybenzene
英文别名
1,4-diethoxy-2-chloro-benzene;1,4-Diaethoxy-2-chlor-benzol;1-chloro-2,5-diethoxybenzene
CAS
52196-74-4
化学式
C10H13ClO2
mdl
MFCD00026765
分子量
200.665
InChiKey
ZIMKMIAIVORSSX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:15 °C
-
沸点:266 °C
-
密度:1.14
-
闪点:130°C(lit.)
-
保留指数:1442.5
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S16
-
危险类别码:R10
-
RTECS号:QL3854000
-
海关编码:2909309090
-
危险品运输编号:1325
SDS
1-氯-2,5-二乙氧基苯 修改号码:5
模块 1. 化学品
产品名称: 1-Chloro-2,5-diethoxybenzene
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-氯-2,5-二乙氧基苯
百分比: >98.0%(GC)
CAS编码: 52196-74-4
俗名: 2,5-Diethoxychlorobenzene
分子式: C10H13ClO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
1-氯-2,5-二乙氧基苯 修改号码:5
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 极淡的黄色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 266 °C
闪点: 130°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.14
溶解度:
[水] 无资料
[其他溶剂] 无资料
1-氯-2,5-二乙氧基苯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1-氯-2,5-二乙氧基苯 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1-Chloro-2,5-diethoxybenzene
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-氯-2,5-二乙氧基苯
百分比: >98.0%(GC)
CAS编码: 52196-74-4
俗名: 2,5-Diethoxychlorobenzene
分子式: C10H13ClO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
1-氯-2,5-二乙氧基苯 修改号码:5
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 极淡的黄色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 266 °C
闪点: 130°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.14
溶解度:
[水] 无资料
[其他溶剂] 无资料
1-氯-2,5-二乙氧基苯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1-氯-2,5-二乙氧基苯 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 氯氢醌 Chlorohydroquinone 615-67-8 C6H5ClO2 144.558 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氯-2,5-二乙氧基苯胺 4-chloro-2,5-diethoxyaniline 35271-60-4 C10H14ClNO2 215.68
反应信息
-
作为反应物:描述:2-氯-1,4-二乙氧基苯 在 HNO3-SiO2 、 palladium 10% on activated carbon 、 氢气 作用下, 以 甲醇 、 二氯甲烷 、 乙酸乙酯 为溶剂, 反应 16.0h, 生成 1-chloro-2,5-diethoxy-4-isothiocyanatobenzene参考文献:名称:Structure–activity relationship studies of 1-(4-chloro-2,5-dimethoxyphenyl)-3-(3-propoxypropyl)thiourea, a non-nucleoside reverse transcriptase inhibitor of human immunodeficiency virus type-1摘要:The reverse transcriptase (RT) of the human immunodeficiency virus type-1 (HIV-1) is still a prime target for drug development due to the continuing need to block drug-resistant RT mutants by new inhibitors. We have previously identified 1-(4-chloro-2,5-dimethoxyphenyl)-3-(3-propoxypropyl)thiourea, compound 1, as a potent RI inhibitor from an available chemical library. Here, we further modified this compound to study structure activity relationships when replacing various groups in the molecule. Different functional groups were systematically introduced on the aromatic ring and the aliphatic chain of the compound was modified. The effect of these modifications on viral infectivity was then evaluated. The most potent compound found was propyl 4-(amino-N-(4-chloro-2,5-dimethoxyphenyl)methanethioamino)butanoate, 45c, which inhibited infectivity with a calculated IC50 of about 1.1 mu M. Docking studies identified potential important interactions between the top scoring ligands and HIV-1 RT, and the predicted relative affinity of the ligands was found to be in agreement with the experimental results. (C) 2010 Elsevier Masson SAS. All rights reserved.DOI:10.1016/j.ejmech.2010.11.003
-
作为产物:描述:参考文献:名称:Klaassens; Schoot, Recueil des Travaux Chimiques des Pays-Bas, 1953, vol. 72, p. 91摘要:DOI:
文献信息
-
Sur les quinones dérivées du diphényle formées par oxydation d'éthers de benzohydroquinones substiuées作者:Th. Posternak、W. Alcalay、R. Luzzati、A. TardentDOI:10.1002/hlca.19480310232日期:——Les quinones obtenues par oxydation d'éthers de benzhydroquinones substituées dérivent de la diphénoquinone-3,6 et noncomme on I'avait indiqué autrefois, de la diphénoquinone-6,6′.
-
METHOD FOR PRODUCING BIARYL COMPOUND申请人:Sato Koichi公开号:US20100087680A1公开(公告)日:2010-04-08A method for producing a biaryl compound, comprising reacting an aromatic organic compound with at least one compound selected from the group consisting of aromatic organoboron compounds and boroxine compounds, in the presence of a zero-valent nickel catalyst, phosphine ligand and base.
-
PROCESS FOR PRODUCING BIARYL COMPOUND申请人:Sumitomo Chemical Company, Limited公开号:EP1914221A1公开(公告)日:2008-04-23A method for producing a biaryl compound, comprising reacting an aromatic organic compound with at least one compound selected from the group consisting of aromatic organoboron compounds and boroxine compounds, in the presence of a zero-valent nickel catalyst, phosphine ligand and base.
-
Solid‐State Emissive Pillar[6]arene Derivative Having Alternate Methylene and Nitrogen Bridges作者:Shunsuke Ohtani、Kazeto Nakaguchi、Kenichi Kato、Tomoki OgoshiDOI:10.1002/asia.202400106日期:2024.5.2Herein, we synthesized pillar[6]arene derivatives having alternate methylene and nitrogen bridges. Owing to the charge transfer emission, the solid-state photoluminescence quantum yield (ΦPL) was enhanced compared with that of the parent pillar[6]arene (ΦPL=0.063→ΦPL=0.36). Furthermore, it displayed a turn-off sensing toward nitrobenzene (NB) vapor; a fluorescence quenching was observed when exposed
-
Asymmetric Synthesis of α-Aryl Amino Acids; Aryne-Mediated Diastereoselective Arylation作者:Elizabeth P. Jones、Peter Jones、Anthony G. M. BarrettDOI:10.1021/ol1030469日期:2011.3.4An aryne-mediated alpha-arylation reaction of Schollkopf's bis-lactim ether is described. Arynes were generated via an ortho-lithiation approach, affording syn-arylated products in up to 94:6 dr with moderate to good yields and excellent regioselectivities. Hydrolysis provided a variety of substituted arylglycines containing a range of functional groups without racemization.
表征谱图
-
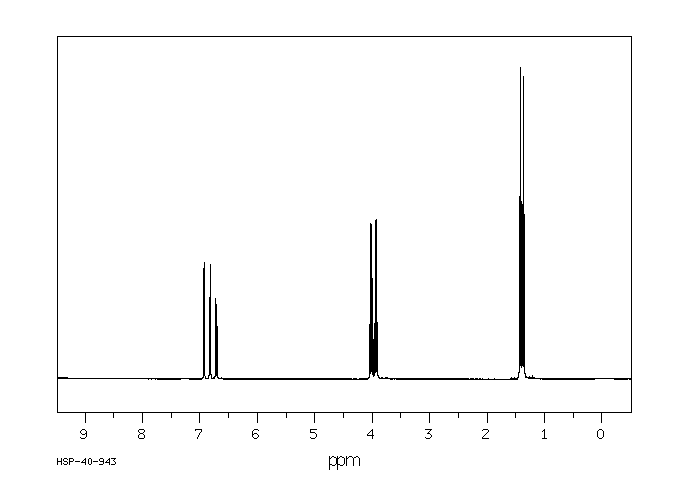
氢谱1HNMR
-
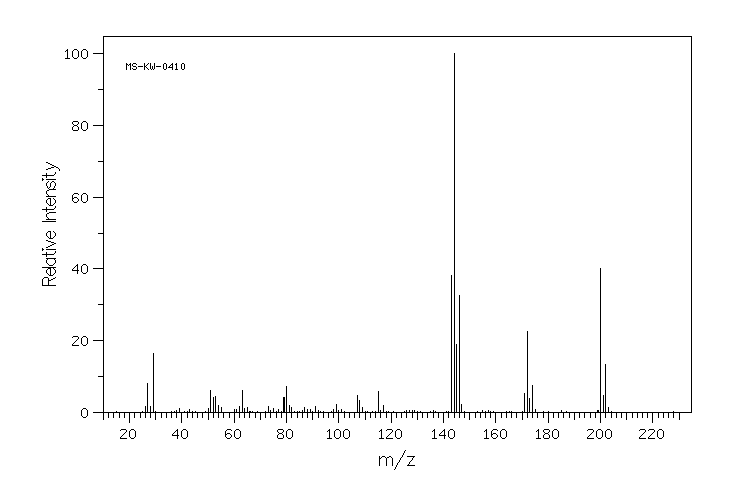
质谱MS
-
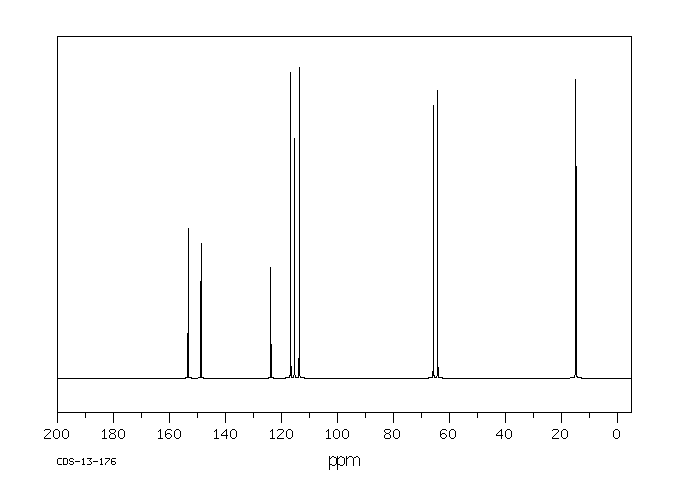
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯