9-己基蒽 | 33576-55-5
中文名称
9-己基蒽
中文别名
——
英文名称
9-Hexylanthracene
英文别名
——
CAS
33576-55-5
化学式
C20H22
mdl
——
分子量
262.395
InChiKey
FFQDRQHAHQLKBE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):7.7
-
重原子数:20
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:9-己基蒽 在 盐酸 、 18-冠醚-6 、 potassium carbonate 、 溶剂黄146 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 17.0h, 生成 3,5-bis[(10-hexylanthracen-9-yl)methoxy]benzyl benzoate参考文献:名称:Synthesis and characterization of anthracene-clustering dendrimers: observation of fluorescence resonance energy transfer in the multichromophoric system摘要:A series of anthracene-clustering dendrimers bearing various aliphatic substituents at the terminal positions were synthesized using a direct coupling strategy. A remarkable effect of the side chains was imparted to chemical properties of the dendrimers such as drastically increased solubility. Although the multibranched anthracene arrays in the dendritic architectures exhibited no cooperativity in terms of the absorption feature and behaved as single chromophoric systems, investigations focusing on fluorescence properties revealed that a type of cooperativity was present as expressed in the reduced quantum yields of fluorescence. An alternative approach utilizing time-resolved fluorescence decay measurements clearly demonstrated that the most reasonable mechanism of the cooperative action should involve two discernible channels of intramolecular fluorescence resonance energy transfer (FRET) occurring from one chromophore to the others within and across junctions of the branching units. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2004.09.096
-
作为产物:参考文献:名称:Conjugate Addition of Grignard Reagents to Nitroarenes: A New Synthesis of 9-Alkylanthracenes, 9-Nitro-10-alkylanthracenes, and 10,10-Dialkylanthrones摘要:DOI:10.1055/s-1982-29963
文献信息
-
Photoinduced double perfluoroalkylation of methylacenes作者:Emiko Nogami、Yuri Washimi、Takashi Yamazaki、Toshio Kubota、Tomoko YajimaDOI:10.1016/j.tetlet.2016.05.001日期:2016.6Novel photoinduced double perfluoroalkylation of methylacenes has been described. The reaction proceeded with appropriately substituted methylacenes to produce a mixture of the expected aromatic compounds with Rf groups both at the methyl and its para position as well as the corresponding nonaromatic tautomers. Steric repulsive interaction of fluorine atoms in perfluoroalkyl groups with hydrogen atoms
-
Synthesis of Conjugated Polyenes with Alkylanthryl and N-Alkylpyridinium Terminal Groups作者:Franz Effenberger、Stephan Heid、Rotraut Merkle、Peter ZimmermannDOI:10.1055/s-1995-4059日期:1995.9The synthesis of polyenes with 3 or 5 conjugated double bonds bearing alkylanthryl and alkylpyridinium terminal groups is described. Alkyl chains of suitable length were introduced in both the anthracene and/or pyridine moiety to reduce the solubility in water thus improving the ability to form Langmuir-Blodgett films.
-
In Continuo Pd‐Catalysed Cross Coupling Reactions of Organolithium Reagents with Aryl Bromides Under Aerobic Conditions作者:Jacopo Brucoli、Davide Gariboldi、Alessandra Puglisi、Sergio Rossi、Vito Capriati、Maurizio BenagliaDOI:10.1002/ejoc.202301289日期:2024.2.12The direct use of readily available and relatively inexpensive organolithium reagents in cross-coupling reactions is grown in recent years. However, the large-scale application of these commodity reagents remains a formidable challenge. Here we report fast, efficient, and in continuo Pd-catalysed cross couplings of organolithium reagents with aryl bromides that proceed in 40 s, allowing the synthesis
-
Substituent effect in the photodimerization of amphiphilic anthracenes: reactions in monolayer assemblies and in solutions作者:Akihiko Ouchi、Hiroyuki Niino、Yasujiro Kawabata、Motoo Tanaka、Akira YabeDOI:10.1016/s0040-4039(00)98024-9日期:1990.1
-
ARMILLOTTA, N.;BARTOLI, G.;BOSCO, M.;DALPOZZO, R., SYNTHESIS, BRD, 1982, N 10, 836-839作者:ARMILLOTTA, N.、BARTOLI, G.、BOSCO, M.、DALPOZZO, R.DOI:——日期:——
表征谱图
-
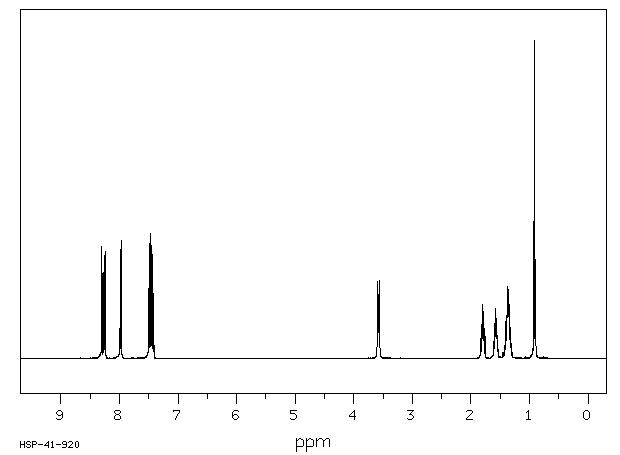
氢谱1HNMR
-
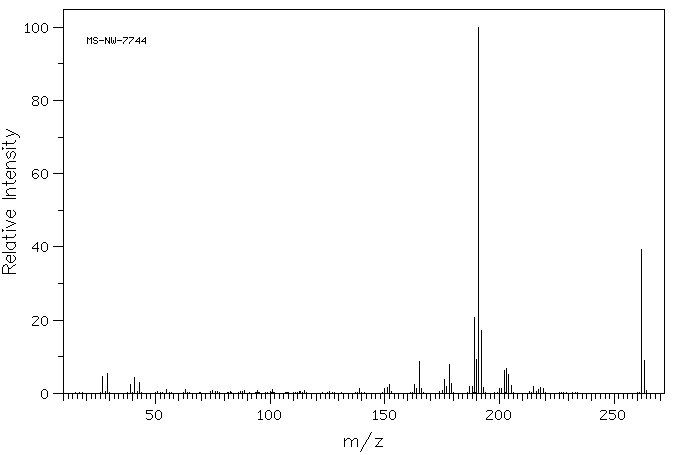
质谱MS
-
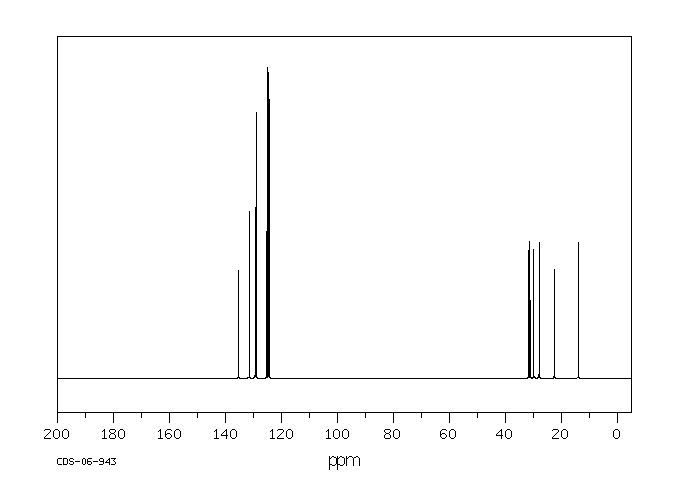
碳谱13CNMR
-
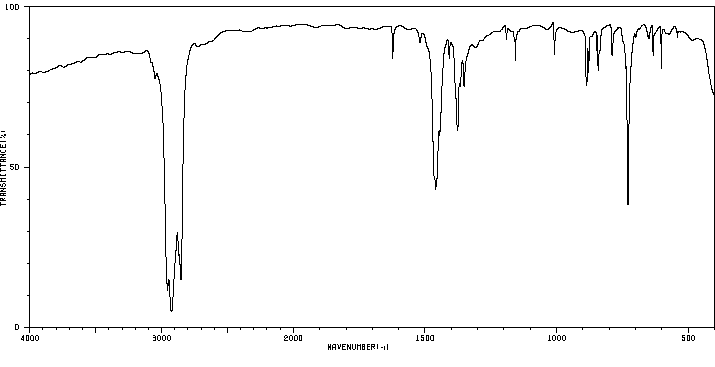
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62