N,N'-二(9,10-二氢-9,10-二氧代-1,5-蒽二基)二-乙酰胺 | 129-30-6
中文名称
N,N'-二(9,10-二氢-9,10-二氧代-1,5-蒽二基)二-乙酰胺
中文别名
1,5-双(乙酰氨基)蒽醌
英文名称
1,5-Diacetamidoanthraquinone
英文别名
1,5-bis-acetylamino-anthraquinone;1,5-Bis-acetylamino-anthrachinon;1.5-Bis-acetamino-anthrachinon;N,N'-(9,10-dioxo-1,5-anthrylene)di(acetamide);1,5-bis(acetamido)-anthraquinone;1,5-Bis-acetaminoanthrachinon;Acetamide, N,N'-bis(9,10-dihydro-9,10-dioxo-1,5-anthracenediyl)bis-;N-(5-acetamido-9,10-dioxoanthracen-1-yl)acetamide
CAS
129-30-6
化学式
C18H14N2O4
mdl
——
分子量
322.32
InChiKey
FKRYJILJIVPKTR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:24
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:92.3
-
氢给体数:2
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,5-二氨基蒽醌 1,5-diaminoanthraquinone 129-44-2 C14H10N2O2 238.246 1,5-二硝基蒽醌 1.5-dinitroanthraquinone 82-35-9 C14H6N2O6 298.211 1-硝基蒽醌 1-nitroanthraquinone 82-34-8 C14H7NO4 253.214 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(5-乙酰氨基-4,8-二硝基-9,10-二氧代蒽-1-基)乙酰胺 1,5-bis-acetylamino-4,8-dinitro-anthraquinone 37686-98-9 C18H12N4O8 412.315 1,5-二氨基-4,8-二硝基蒽-9,10-二酮 1,5-diamino-4,8-dinitro-anthraquinone 10262-79-0 C14H8N4O6 328.241
反应信息
-
作为反应物:描述:参考文献:名称:DE127780摘要:公开号:
-
作为产物:描述:参考文献:名称:Roemer, Chemische Berichte, 1883, vol. 16, p. 363摘要:DOI:
文献信息
-
Synthesis and Antitumor Evaluation of Symmetrical 1,5-Diamidoanthraquinone Derivatives as Compared to Their Disubstituted Homologues作者:Hsu-Shan Huang、Hui-Fen Chiu、Chi-Wei Tao、In-Been ChenDOI:10.1248/cpb.54.458日期:——A series of symmetrical 1,5-diamidoanthraquinone derivatives with potentially bioreducible groups has been synthesized and their cytostatic activity against the panel of various cancer cell lines in vitro has been studied. Preliminary structure–activity relationships were established. The results indicated that compounds 5 and 18 exhibited significant potent cytotoxicity at 1.24—1.75 μM for Hepa G2 cell line; compounds 5, 16, and 18 exhibited cytotoxicity at 0.14—1.82 μM for 2.2.15 cell line as determined by XTT colorimetric assay. Two structurally related compounds, mitoxantrone and adriamycin, were tested in parallel as positive controls. In addition, it was found that compounds 5 and 18 were a more potent and specific human hepatoma cell line than mitoxantrone and showed comparable activity to adriamycin. Among them, compound 18 was the most potent for 2.2.15 cells. We have demonstrated that the anthraquinone moiety is essential for activity and that less sterically hindered substituents contribute to enhanced in vitro efficacy. Implications for amidoanthraquinone cytotoxicity as potential anticancer agents are discussed. We further delineate the nature of the pharmacophore for this class of compounds, which provides a rational basis for the structure–activity relationships.一系列对称的1,5-二氨基蒽醌衍生物已被合成,并研究了其对多种癌细胞系的细胞抑制活性。初步的结构-活性关系得到了建立。结果显示,化合物5和18在1.24-1.75 μM时对Hepa G2细胞系表现出显著的细胞毒性;化合物5、16和18在0.14-1.82 μM时对2.2.15细胞系表现出细胞毒性,通过XTT比色法进行了测定。两种结构相关的化合物,米托蒽醌和阿霉素,被作为阳性对照进行平行测试。此外,发现化合物5和18在对人肝癌细胞系的活性上比米托蒽醌更强,而与阿霉素的活性相当。在上述化合物中,化合物18对2.2.15细胞的活性最强。我们证明了蒽醌基团对于活性是必不可少的,且较少空间位阻的取代基有助于增强体外活性。讨论了氨基蒽醌类化合物作为潜在抗癌剂的细胞毒性影响。我们进一步阐明了这一类化合物的药效基团的性质,为结构-活性关系提供了合理依据。
-
Selective photoalkylamination and photohydroxylation of aminoanthraquinones and their N-acylated derivatives作者:Katsuhira Yoshida、Masahiro Hikasa、Katsutoshi Ishii、Hisa Kadota、Yoshio YamashitaDOI:10.1039/c39860000758日期:——The selective introduction of hydroxy and Alkylamino groups into the anthraquinone nucleus was achieved by the photochemical reaction of some aminoanthraquinones and their N-acylated derivatives with alkylamines under aerobic conditions.
-
Scholl; Wanka, Chemische Berichte, 1929, vol. 62, p. 1426作者:Scholl、WankaDOI:——日期:——
-
DE199758申请人:——公开号:——公开(公告)日:——
-
USE OF ANTHRAQUINONE DYES AND OF FLUORESCENT DYES FOR DYEING KERATIN FIBRES, DYEING PROCESS AND COMPOSITION申请人:L'Oréal公开号:EP3558235B1公开(公告)日:2021-09-08
表征谱图
-
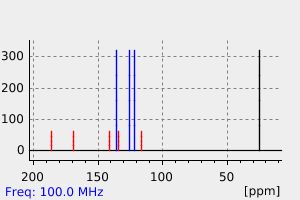
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62