DL-亮氨酰-DL-缬氨酸 | 35436-83-0
中文名称
DL-亮氨酰-DL-缬氨酸
中文别名
——
英文名称
DL-Leu-DL-Val
英文别名
N-leucyl-valine;N-Leucyl-valin;leucylvaline;Leu-Val;Leu-DL-Val;2-[(2-azaniumyl-4-methylpentanoyl)amino]-3-methylbutanoate
CAS
35436-83-0;100758-58-5
化学式
C11H22N2O3
mdl
MFCD00068371
分子量
230.307
InChiKey
MDSUKZSLOATHMH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):-2
-
重原子数:16
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.818
-
拓扑面积:92.4
-
氢给体数:3
-
氢受体数:4
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-异丙基-6-(2-甲基-丙基)-2,5-哌嗪二酮 cyclo-(Leu-Val) 5625-50-3 C11H20N2O2 212.292
反应信息
-
作为反应物:描述:参考文献:名称:Abderhalden; Gebelein, Hoppe-Seyler's Zeitschrift fur Physiologische Chemie, 1926, vol. 152, p. 131摘要:DOI:
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 氨 作用下, 生成 DL-亮氨酰-DL-缬氨酸参考文献:名称:Abderhalden; Sickel, Fermentforschung, vol. 9, p. 469摘要:DOI:
文献信息
-
DRY POWDER INHALATION SYSTEM FOR TRANSPULMONARY ADMINISTRATION申请人:OTSUKA PHARMACEUTICAL CO., LTD.公开号:EP1402913A1公开(公告)日:2004-03-31The present invention provides a novel dry powder inhalation system suitable for transpulmonary administration. The dry powder inhalation system of the invention characterized by using a combination of: (1) a vessel housing a freeze-dried composition that contains a single dose of an active ingredient, and has: (i) a non-powder cake-like form, (ii) a disintegration index of 0.015 or more, and (iii) a property of becoming fine particles having a mean particle diameter of 10 microns or less or a fine particle fraction of 10% or more upon receipt of an air impact having an air speed of at least 1 m/sec and an air flow rate of at least 17 ml/sec; and (2) a device comprising means capable of applying said air impact to the freeze-dried composition in said vessel, and means for discharging the powder-form freeze-dried composition that has been made into fine particles.本发明提供了一种适用于跨肺给药的新型干粉吸入系统。本发明的干粉吸入系统的特点是采用了以下组合: (1) 一个容纳冷冻干燥组合物的容器,该组合物含有单剂量的活性成分,并具有以下特征 (i) 非粉饼状、 (ii) 崩解指数为 0.015 或以上,以及 (iii) 在受到气速至少为 1 米/秒和气流速率至少为 17 毫升/秒的空气冲击时,成为平均粒径为 10 微米或以下或细颗粒分数为 10%或以上的细颗粒的特性;以及 (iv) 在受到气速至少为 1 米/秒和气流速率至少为 17 毫升/秒的空气冲击时,成为平均粒径为 10 微米或以下或细颗粒分数为 10%或以上的细颗粒的特性。 (2) 一种装置,包括能够对所述容器中的冻干成分施加所述空气冲击的装置,以及将已制成细颗粒的粉末状冻干成分排出的装置。
-
Dry compositions comprising hydrophobic stabilisers申请人:OTSUKA PHARMACEUTICAL CO., LTD.公开号:EP1424066A1公开(公告)日:2004-06-02The object of the present invention is to provide a dry composition having the following advantageous properties. That is, even when left in a highly humid environment, the dry composition of the present invention scarcely loses its pharmacological activity, does not deliquesce and retains its dry state over a long period of time. A dry composition of the present invention comprises at least one of active ingredients selected from the group consisting of pharmacologically active proteins and pharmacologically active polypeptides and as a stabilizer at least one of hydrophobic stabilizers selected from the group consisting of hydrophobic amino acids, hydrophobic dipeptides and hydrophobic tripeptides.
-
NOVEL DRY POWDER INHALATION SYSTEM FOR TRANSPULMONARY ADMINISTRATION申请人:OTSUKA PHARMACEUTICAL CO., LTD.公开号:EP1579855A1公开(公告)日:2005-09-28The present invention provides a novel dry powder inhalation system suitable for transpulmonary administration. The dry powder inhalation system of the present invention characterized by using a combination of: (1) a vessel housing a freeze-dried composition prepared by freeze-drying a composition liquid containing ingredients in a non-dissolved form, and has: (i) a non-powder cake-like form, (ii) a disintegration index of 0.05 or more, and (iii) a property of becoming fine particles having a mean particle diameter (mass median aerodynamic diameter) of 10 microns or less or a fine particle fraction of 10% or more upon receipt of an air impact having an air speed of at least 1 m/sec and an air flow rate of at least 17 ml/sec; and (2) a device comprising a member capable of applying said air impact to the freeze-dried composition in said vessel, and a member for discharging the powder-form freeze-dried composition that has been made into fine particles.本发明提供了一种适用于跨肺给药的新型干粉吸入系统。本发明的干粉吸入系统的特点是使用以下组合: (1) 容器,容纳通过冷冻干燥含有未溶解成分的组合物液体而制备的冷冻干燥组合物,并具有以下特点 (i)非粉饼状,(ii)崩解指数为 0.05 或更高,和(iii)在接受气速至少为 1 米/秒和空气流速至少为 17 毫升/秒的空气冲击时,具有成为平均颗粒直径(质量中值空气动力学直径)为 10 微米或更小或细颗粒分数为 10%或更高的细颗粒的特性;和 (2) 一种装置,包括一个能够对所述容器中的冻干成分施加所述空气冲击的部件,以及一个用于排出已制成细颗粒的粉末状冻干成分的部件。
-
Freeze-dried composition for transpulmonary administration申请人:OTSUKA PHARMACEUTICAL CO., LTD.公开号:EP1688133A1公开(公告)日:2006-08-09A freeze-dried composition for transpulmonary administration having the following properties (i) to (iii): (i) has a non-powder cake-like form, (ii) has a disintegration index of 0.015 or more, and (iii) becomes fine particles having a mean particle diameter of 10 microns or less or a fine particle fraction of 10% or more upon receipt of an air impact having an air speed of at least 1m/sec and an air flow rate of at least 17ml/sec.具有以下特性(i)至(iii)的经肺给药冻干组合物: (i) 不呈粉饼状、 (ii) 崩解指数为 0.015 或以上,以及 (iii) 在受到气速至少为 1 米/秒、气流速率至少为 17 毫升/秒的空气冲击时,变成平均粒径为 10 微米或以下或细粒率为 10%或以上的细颗粒。
-
Dry powder inhaler for transpulmonary administration申请人:OTSUKA PHARMACEUTICAL CO., LTD.公开号:EP1688134A2公开(公告)日:2006-08-09A dry powder inhaler for transpulmonary administration used for making a freeze-dried composition that has been housed in non-powder form in a vessel into fine particles by an air impact, and administering the resulting fine particles to a user by inhalation, comprising a needle part having an air jet flow path, a needle part having a discharge flow path, air pressure-feeding means for feeding air into the air jet flow path of said needle part, and an inhalation port that communicates with the discharge flow path of said needle part, and being constituted such that a stopper that seals up said vessel is pierced by said needle parts, thus communicating the air jet flow path and the discharge flow path with the inside of said vessel, and air is jetted into said vessel through said air jet flow path using said air pressure-feeding means, thus making said freeze-dried composition into fine particles by the impact of the jetted air, and discharging the fine particles from the inhalation port via said discharge flow path.一种用于跨肺给药的干粉吸入器,用于通过空气冲击将容器中的非粉末状冻干成分制成细小颗粒,并通过吸入将所得细小颗粒提供给使用者、 包括一个具有空气喷射流路的针头部件、一个具有排放流路的针头部件、用于将空气送入所述针头部件的空气喷射流路的空气压力供给装置,以及一个与所述针头部件的排放流路相通的吸入口、 其构成为,密封所述容器的塞子被所述针状部件刺穿,从而使空气喷射流路和排出流路与所述容器内部相通,利用所述空气压力供给装置通过所述空气喷射流路向所述容器喷射空气,从而通过喷射空气的冲击力将所述冻干成分制成细小颗粒,并通过所述排出流路将细小颗粒从吸入口排出。
表征谱图
-
氢谱1HNMR
-
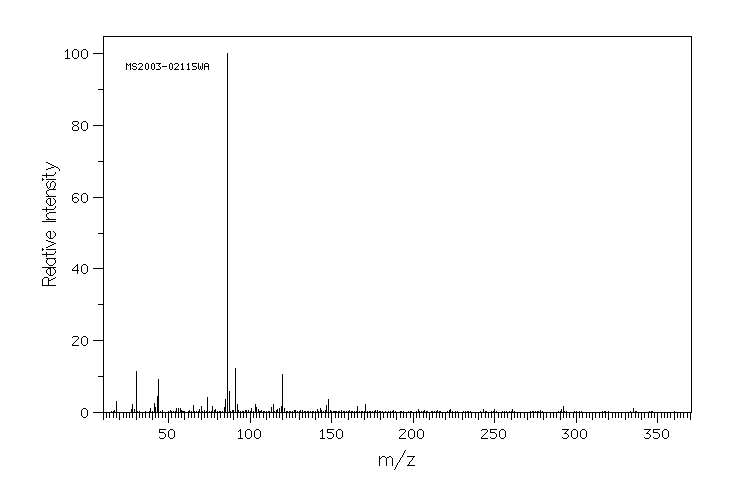
质谱MS
-
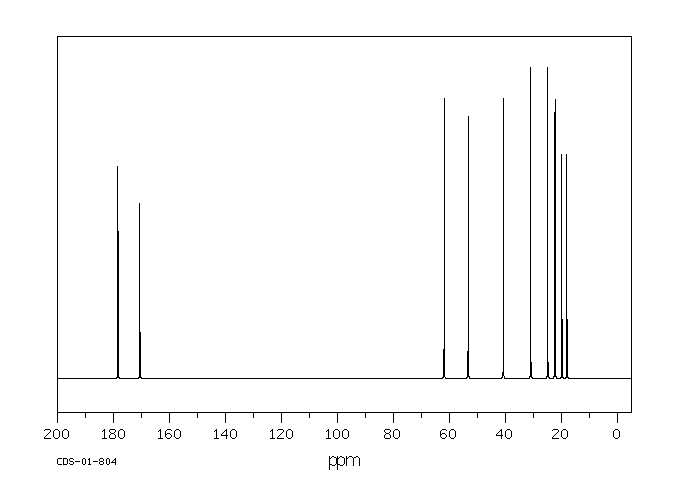
碳谱13CNMR
-
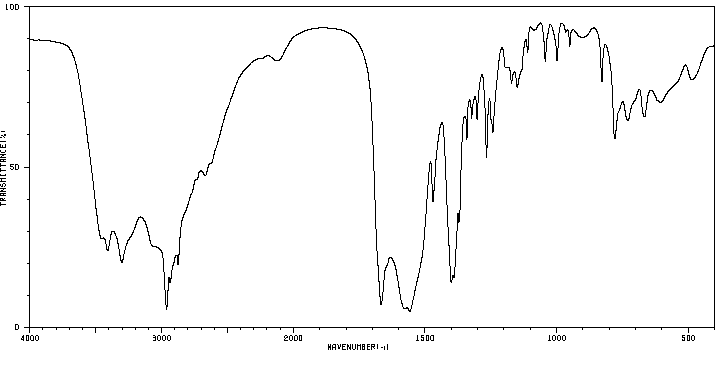
红外IR
-
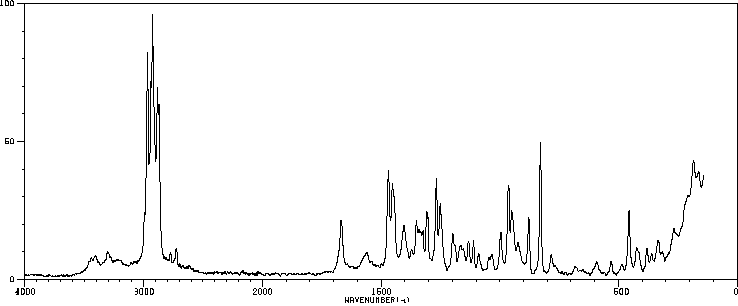
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸