全氢化芴 | 5744-03-6
中文名称
全氢化芴
中文别名
全氢芴;十二氢芴(异构体混合物);十二氢芴
英文名称
dodecahydrofluorene
英文别名
Perhydrofluoren;perhydrofluorene;2,3,4,4a,4b,5,6,7,8,8a,9,9a-dodecahydro-1H-fluorene
CAS
5744-03-6
化学式
C13H22
mdl
MFCD00001161
分子量
178.318
InChiKey
OLWAZOBRCQWWDB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:15°C(lit.)
-
沸点:253 °C(lit.)
-
密度:0.92 g/mL at 25 °C(lit.)
-
闪点:148 °F
-
介电常数:2.3100000000000001
-
保留指数:1466;1489;1430;1455;1442;1464;1447
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:13
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xn
-
危险类别码:R22
-
WGK Germany:3
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Perhydrofluorene
CAS-No. : 5744-03-6
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Oral (Category 4)
Eye irritation (Category 2)
Chronic aquatic toxicity (Category 4)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Harmful if swallowed.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
Harmful if swallowed.
Causes serious eye irritation.
May cause long lasting harmful effects to aquatic life.
Precautionary statement(s)
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R22 Harmful if swallowed.
S-phrase(s) none
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : Dodecahydrofluorene
Formula : C13H22
Molecular Weight : 178,31 g/mol
Component Concentration
Perhydrofluoren
CAS-No. 5744-03-6 -
EC-No. 227-268-3
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
For small (incipient) fires, use media such as "alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large quantities (flooding) of water applied as a mist or
spray; solid streams of water may be ineffective. Cool all affected containers with flooding quantities of
water.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
Use water spray to cool unopened containers.
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapors, mist or gas. Ensure adequate ventilation.
Remove all sources of ignition. Beware of vapours accumulating to form explosive concentrations.
Vapours can accumulate in low areas.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the
environment must be avoided.
Methods and materials for containment and cleaning up
Contain spillage, and then collect with an electrically protected vacuum cleaner or by wet-brushing and
place in container for disposal according to local regulations (see section 13). Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Keep away from sources of ignition - No smoking.Take measures to prevent the build up of electrostatic
charge.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 253 °C - lit.
boiling range
g) Flash point 64 °C - closed cup
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 0,92 g/cm3 at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 6,12
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
Heat, flames and sparks.
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
Prolonged or repeated exposure may cause allergic reactions in certain sensitive individuals.
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes Causes eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
This combustible material may be burned in a chemical incinerator equipped with an afterburner and
scrubber. Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine Pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,3,5-三甲基金刚烷 1,3,5-trimethyladamantane 707-35-7 C13H22 178.318
反应信息
-
作为反应物:参考文献:名称:The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage摘要:The effect of the N atom on the hydrogen release rate from heterocyclic compounds was studied by comparing the dehydrogenation rate of dodecahydro-N-ethylcarbazole, dodecahydrocarbazole and dodecahydrofluorene. Over a 5 wt% Pd/C catalyst, hydrogen recovery was fastest (TOF 60 min(-1) at 443 K and 101 kPa) from dodecahydro-N-ethylcarbazole and similar to 3 times faster than that of dodecahydrocarbazole. Dodecahydrofluorene dehydrogenation was the slowest among the compounds examined, with less than 1 wt% H-2 recovered after more than 20 h at 443 K, although selectivity to the completely dehydrogenated product was 95%. Despite catalyst poisoning by the N in dodecahydrocarbazole and its dehydrogenated product, the presence of the N in the heterocycle increased the dehydrogenation reaction rate compared to dodecahydrofluorene, demonstrating that heterocycles are better candidates for H-2 storage than polycycles. (C) 2012 Elsevier B.V. All rights reserved.DOI:10.1016/j.apcata.2012.01.013
-
作为产物:参考文献:名称:用碳和2-丙醇上的铂结合氢自足芳烃还原成环己烷衍生物摘要:在不添加易燃氢气的情况下,使用2-碳丙醇-水混合溶剂中的碳载铂在不添加易燃氢气的情况下,将各种芳烃进行了氢化,得到了相应的环己烷衍生物。2-丙醇在碳催化脱氢的基础上,起着基于铂的高效氢源的作用。DOI:10.1002/adsc.201500263
-
作为试剂:描述:参考文献:名称:Bott,K., Chemische Berichte, 1970, vol. 103, p. 3850 - 3861摘要:DOI:
文献信息
-
Titanium(III)-Oxo Clusters in a Metal–Organic Framework Support Single-Site Co(II)-Hydride Catalysts for Arene Hydrogenation作者:Pengfei Ji、Yang Song、Tasha Drake、Samuel S. Veroneau、Zekai Lin、Xiandao Pan、Wenbin LinDOI:10.1021/jacs.7b11241日期:2018.1.10clusters for supporting single-site catalysts. Herein we report that the Ti8(μ2-O)8(μ2-OH)4 node of the Ti-BDC MOF (MIL-125) provides a single-site model of the classical TiO2 support to enable CoII-hydride-catalyzed arene hydrogenation. The catalytic activity of the supported CoII-hydride is strongly dependent on the reduction of the Ti-oxo cluster, definitively proving the pivotal role of TiIII in the performance二氧化钛 (TiO2) 因其独特的强金属-载体相互作用而在化学工业中广泛用作有效的催化剂载体。已经提出了许多建议在宏观水平上合理化这种影响,但由于 TiO2 表面上存在多种催化物质,潜在的分子机制尚不清楚。这一挑战可以通过金属有机框架 (MOF) 来解决,该框架具有明确定义的金属氧/羟基团簇,用于支持单中心催化剂。在此,我们报告了 Ti-BDC MOF (MIL-125) 的 Ti8(μ2-O)8(μ2-OH)4 节点提供了经典 TiO2 载体的单中心模型,以实现 CoII-氢化物催化的芳烃氢化. 负载型 CoII-氢化物的催化活性强烈依赖于 Ti-oxo 簇的还原,最终证明了 TiIII 在负载型催化剂性能中的关键作用。因此,这项工作提供了 Ti-oxo 簇的分子精确模型,用于低估 TiO2 负载的多相催化剂的强金属-载体相互作用。
-
Efficient and Practical Arene Hydrogenation by Heterogeneous Catalysts under Mild Conditions作者:Tomohiro Maegawa、Akira Akashi、Kiichiro Yaguchi、Yohei Iwasaki、Masahiro Shigetsura、Yasunari Monguchi、Hironao SajikiDOI:10.1002/chem.200900361日期:2009.7.13An efficient and practical arene hydrogenation procedure based on the use of heterogeneous platinum group catalysts has been developed. Rh/C is the most effective catalyst for the hydrogenation of the aromatic ring, which can be conducted in iPrOH under neutral conditions and at ordinary to medium H2 pressures (<10 atm). A variety of arenes such as alkylbenzenes, benzoic acids, pyridines, furans, are
-
The standard molar enthalpies of formation of some alkyladamantanes作者:S.V. Melkhanova,、S.M. Pimenova,、V.P. Kolesov,、A.A. Pimerzin,、V.S. SarkisovaDOI:10.1006/jcht.2000.0691日期:2000.10energies of combustion of three alkyl-derivative of adamantane were measured atT = 298.15 K by static-bomb combustion calorimetry. The standard molar enthalpies of formation in the liquid and gaseous states were obtained from these data. The enthalpies of some reactions of isomerization were calculated from the equilibrium study and compared with the results of calorimetric measurements.
-
Acenaphthene and fluorene hydrogenation on industrial aluminum oxide catalysts in a flow system作者:E. I. Bagrii、M. V. TsodikovDOI:10.1134/s0965544114020029日期:2014.3Hydrogenation of the tricyclic aromatic hydrocarbons acenaphthene and fluorene on industrial aluminum oxide catalysts in a flow system has been studied. It has been found that total these hydrocarbons are exhaustively hydrogenated in the presence of a nickel-chromium catalyst at 200°C and a pressure of 100 atm to give isomer mixtures of the corresponding perhydroaromatic hydrocarbons decahydroacenaphthene
-
Reduction of aromatic compounds with Al powder using noble metal catalysts in water under mild reaction conditions作者:Ummey Rayhan、Hyeokmi Kwon、Takehiko YamatoDOI:10.1016/j.crci.2013.09.013日期:2014.9Résumé In water, Al powder becomes a powerful reducing agent, transforming in cyclohexyl either one or both benzene rings of aromatic compounds such as biphenyl, fluorene and 9,10-dihydroanthracene under mild reaction conditions in the presence of noble metal catalysts, such as Pd/C, Rh/C, Pt/C, or Ru/C. The reaction is carried out in a sealed tube, without the use of any organic solvent, at low temperature. Partial aromatic ring reduction was observed when using Pd/C, the reaction conditions being 24 h and 60 °C. The complete reduction process of both aromatic rings required 12 h and 80 °C with Al powder in the presence of Pt/C. Supplementary Materials: Supplementary material for this article is supplied as a separate file: mmc1.pdf
表征谱图
-
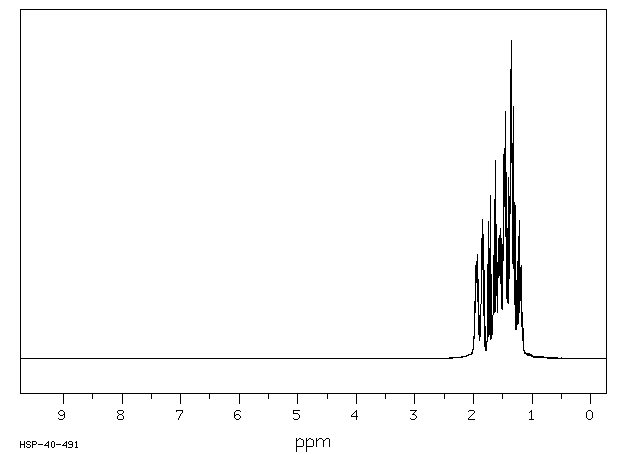
氢谱1HNMR
-
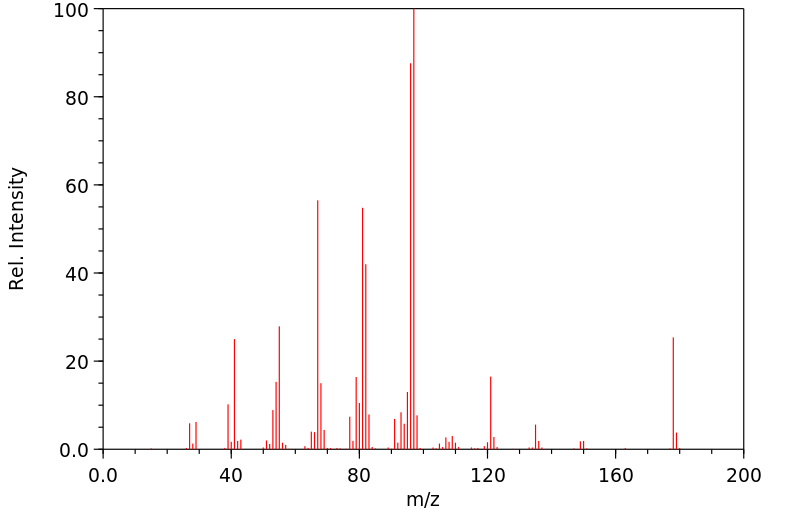
质谱MS
-
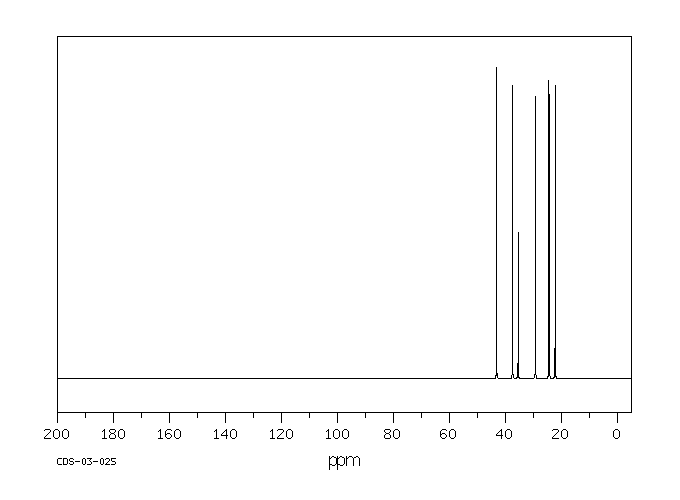
碳谱13CNMR
-
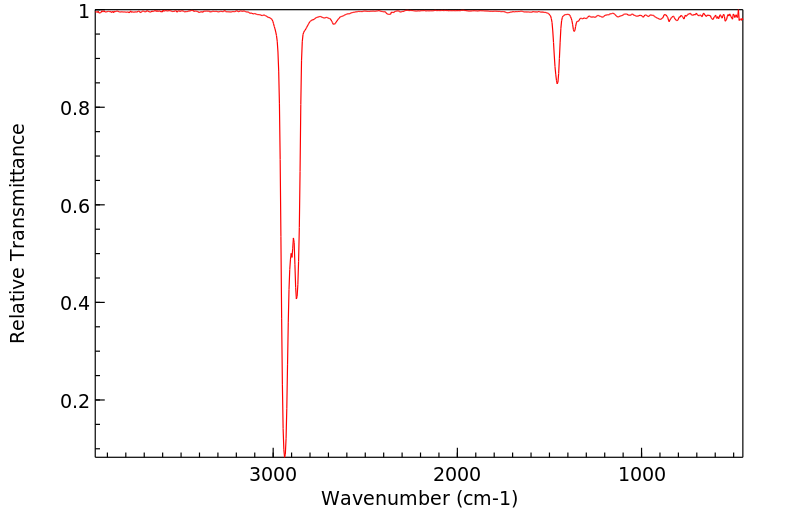
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷