2-甲氧基-1-萘甲醇 | 40696-22-8
中文名称
2-甲氧基-1-萘甲醇
中文别名
1-(羟甲基)-2-甲氧基萘
英文名称
(2-methoxynaphthalen-1-yl)methanol
英文别名
2-methoxy-1-naphthalenemethanol;(2-methoxy-naphthalene-1-yl)-methanol
CAS
40696-22-8
化学式
C12H12O2
mdl
MFCD00274224
分子量
188.226
InChiKey
VBHARLNUEDIKQD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:100-104 °C
-
沸点:283.23°C (rough estimate)
-
密度:1.0750 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
海关编码:2909499000
SDS
| Name: | 2-METHOXY-1-NAPHTHALENEMETHANOL Material Safety Data Sheet |
| Synonym: | |
| CAS: | 40696-22-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 40696-22-8 | 2-METHOXY-1-NAPHTHALENEMETHANOL | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 40696-22-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 101 - 102 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: insoluble
Specific Gravity/Density:
Molecular Formula:
Molecular Weight: 188.23
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 40696-22-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-METHOXY-1-NAPHTHALENEMETHANOL - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 40696-22-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 40696-22-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 40696-22-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基-1-萘醛 2-methoxy-1-naphthaldehyde 5392-12-1 C12H10O2 186.21 1-(氯甲基)-2-甲氧基萘 1-chloromethyl-2-methoxynaphthalene 67367-39-9 C12H11ClO 206.672 2-甲氧基萘-1-甲胺 1-aminomethyl-2-methoxynaphthalene 136402-93-2 C12H13NO 187.241 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基-1-萘醛 2-methoxy-1-naphthaldehyde 5392-12-1 C12H10O2 186.21 1-(氯甲基)-2-甲氧基萘 1-chloromethyl-2-methoxynaphthalene 67367-39-9 C12H11ClO 206.672 —— 1-(bromomethyl)-2-methoxynaphthalene 91571-00-5 C12H11BrO 251.123 —— 1-[[3,5-Bis(phenylmethoxy)phenyl]methoxymethyl]-2-methoxynaphthalene 862806-04-0 C33H30O4 490.599 3-(2-甲氧基萘-1-基)丙-2-烯酸 3-(2-methoxynaphthalen-1-yl)acrylic acid 106276-48-6 C14H12O3 228.247 3-(2-甲氧基-1-萘基)丙酸 3-(2-methoxynaphthalen-1-yl)propanoic acid 34225-11-1 C14H14O3 230.263 —— 2-Methoxy-1-[[3,4,5-tris(phenylmethoxy)phenyl]methoxymethyl]naphthalene 862806-05-1 C40H36O5 596.723
反应信息
-
作为反应物:描述:参考文献:名称:Palladium-catalyzed C–H dimethylamination of 1-chloromethyl naphthalenes with N,N-dimethylformamide as the dimethyl amino source摘要:用 DMF 对 1-氯甲基萘进行远程 C-H 二甲基化。DOI:10.1039/d3ob00600j
-
作为产物:描述:参考文献:名称:613.有机反应中的氢化物离子。第二部分。在酸存在下,通过苯乙酮与醌反应制备苯乙铵盐摘要:DOI:10.1039/jr9630003295
文献信息
-
Surfactant-Type Brønsted Acid Catalyzed Dehydrative Nucleophilic Substitutions of Alcohols in Water作者:Seiji Shirakawa、Shū KobayashiDOI:10.1021/ol062813j日期:2007.1.1A protocol for the dehydrative nucleophilic substitution of benzyl alcohols with a variety of carbon- and heteroatom-centered nucleophiles using dodecylbenzenesulfonic acid (DBSA) as a surfactant-type Bronsted acid catalyst in water has been developed. The reaction system can be applied to the stereoselective C-glycosylation of 1-hydroxy sugars in water. [reaction: see text].
-
[EN] ION CHANNEL ANTAGONISTS/BLOCKERS AND USES THEREOF<br/>[FR] ANTAGONISTES/BLOQUEURS DES CANAUX IONIQUES ET LEURS UTILISATIONS申请人:SHANGHAI EAST HOSPITAL公开号:WO2021114313A1公开(公告)日:2021-06-17Provided are ion channel antagonists/blockers and uses thereof. Specifically, it provides the compounds of formula (I) or pharmaceutically acceptable salts, stereoisomers, solvates or prodrugs, preparation method therefor and application thereof. Definition of each group in the formula can be found in the specification for details. Provided is also pharmaceutical composition useful for treatment of heart disease and other ion channel related diseases.提供了离子通道拮抗剂/阻断剂及其用途。具体而言,提供了式(I)的化合物或药用盐、立体异构体、溶剂合物或前药,其制备方法及应用。每个式中的各个基团的定义可在说明书中找到详细信息。还提供了用于治疗心脏病和其他离子通道相关疾病的药物组合物。
-
Retro-Claisen benzylation: direct use of benzyl alcohols in Pd-catalyzed couplings with nitriles作者:Tapan Maji、Kinthada Ramakumar、Jon A. TungeDOI:10.1039/c4cc07001a日期:——A new strategy has been developed for the benzylation of nitriles directly from benzyl alcohols. In this process benzyl alcohols undergo retro-Claisen activation with cyanoacetic esters to generate an active electrophile and a carbanionic nucleophile. In the presence of Pd(0) these intermediates undergo catalytic coupling to generate a new C-C bond, resulting in the formation of phenyl propionitriles
-
Synthesis of Dendritic Phenol Ether Derivatives with a Naphthalene Core作者:Zhonghua Xiang、Derong CaoDOI:10.1080/00397910801913980日期:2008.4Abstract A series of dendritic phenol ether derivatives with a naphthalene core were synthesized by the Williamson reaction as a coupling reaction between 1‐hydroxymethylnaphthalene and polyether‐based dendritic fragments in the presence of phase‐transfer catalyst and alkali. The modified method for chlorination of dendritic benzyl alcohol was also developed using PPh3 and CCl4 as reagents and CH2Cl2/CCl4
-
Zeolite β Induced Rearrangement of Allyl Benzyl Ethers. 6. Variation of the Aromatic Part and Synthesis of Dihydronaphthalene Derivatives作者:Johan Wennerberg、Fredrik Ek、Anna Hansson、Torbjörn FrejdDOI:10.1021/jo980698n日期:1999.1.1The zeolite beta induced rearrangement of substituted allyl benzyl ethers to give 4-arylbutanals was investigated with respect to the substituents in the aromatic ring. In some cases the resulting aldehydes cyclized spontaneously to give dihydronaphthalene derivatives. The rearrangement and also the ring closure to dihydronaphthalenes failed or gave poor yields in cases where too weak electron-donating
表征谱图
-
氢谱1HNMR
-
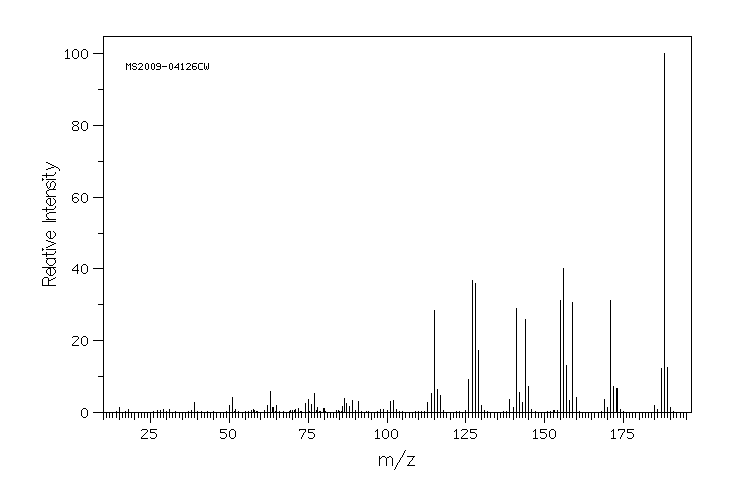
质谱MS
-
碳谱13CNMR
-
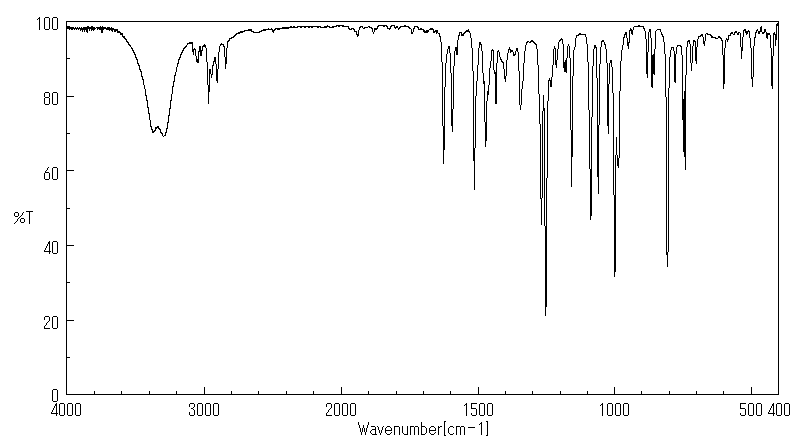
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮