乙酸-2-乙烯基苯基酯 | 63600-35-1
中文名称
乙酸-2-乙烯基苯基酯
中文别名
2-乙烯基苯基乙酸酯;乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
英文名称
2-acetoxystyrene
英文别名
2-vinylphenyl acetate;Acetic Acid 2-Vinylphenyl Ester;(2-ethenylphenyl) acetate
CAS
63600-35-1
化学式
C10H10O2
mdl
——
分子量
162.188
InChiKey
WRPYDXWBHXAKPT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:55-57 °C(Press: 0.4 Torr)
-
密度:1.07
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险类别码:R36/37/38
-
海关编码:2915390090
-
安全说明:S26,S36/37/39
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
SDS
乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
模块 1. 化学品
产品名称: 2-Vinylphenyl Acetate (stabilized with Phenothiazine)
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
百分比: >93.0%(GC)
CAS编码: 63600-35-1
俗名: Acetic Acid 2-Vinylphenyl Ester (stabilized with Phenothiazine) , 2-
Acetoxystyrene (stabilized with Phenothiazine)
乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
模块 3. 成分/组成信息
分子式: C10H10O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷藏储存。
远离不相容的材料比如氧化剂存放。
热敏, 光敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
模块 9. 理化特性
外观: 透明
颜色: 无色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.07
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Vinylphenyl Acetate (stabilized with Phenothiazine)
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
百分比: >93.0%(GC)
CAS编码: 63600-35-1
俗名: Acetic Acid 2-Vinylphenyl Ester (stabilized with Phenothiazine) , 2-
Acetoxystyrene (stabilized with Phenothiazine)
乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
模块 3. 成分/组成信息
分子式: C10H10O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷藏储存。
远离不相容的材料比如氧化剂存放。
热敏, 光敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
模块 9. 理化特性
外观: 透明
颜色: 无色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.07
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
乙酸-2-乙烯基苯基酯(含稳定剂吩噻嗪)
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— benzo[b]oxepin-3(2H)-one 369376-68-1 C10H8O2 160.172
反应信息
-
作为反应物:描述:参考文献:名称:醌的光诱导反应和相关反应。第六部分 一些带有甲酰基的对苯醌的反应摘要:甲酰基-1,4-苯醌,1,4-苯醌基乙醛,3-(1,4-苯醌基)丙醛,2-(1,4-苯醌基)-2-甲基丙醛和2-甲基-2-(1)的制备描述了(4-萘醌-2-基)丙醛。用可见光照射这些苯醌在苯中的溶液导致将2,5-二羟基苯甲酸从甲酰基-1,4-苯醌中分离出来,并形成5-羟基香豆素-2-酮和3,4-二氢-接下来的两个醌中的6-羟基香豆素;该系列中的最后两个醌失去甲醛元素,分别生成5-羟基-3-甲基苯并呋喃和5-羟基-3-甲基萘-[1,2- b ]呋喃。没有任何辐射进行得很干净。DOI:10.1039/j39700000649
-
作为产物:描述:参考文献:名称:Photochemical Reactions. X.1,2 Experiments with 1,3,5-Cycloöctatrien-7-one and Cycloöctatetraene Epoxide摘要:DOI:10.1021/ja00875a014
文献信息
-
Cross-metathesis reaction of functionalized and substituted olefins using group 8 transition metal carbene complexes as metathesis catalysts申请人:CALIFORNIA INSTITUTE OF TECHNOLOGY公开号:US09403854B2公开(公告)日:2016-08-02The invention pertains to the use of Group 8 transition metal carbene complexes as catalysts for olefin cross-metathesis reactions. In particular, ruthenium and osmium alkylidene complexes substituted with an N-heterocyclic carbene ligand are used to catalyze cross-metathesis reactions to provide a variety of substituted and functionalized olefins, including phosphonate-substituted olefins, directly halogenated olefins, 1,1,2-trisubstituted olefins, and quaternary allylic olefins. The invention further provides a method for creating functional diversity using the aforementioned complexes to catalyze cross-metathesis reactions of a first olefinic reactant, which may or may not be substituted with a functional group, with each of a plurality of different olefinic reactants, which may or may not be substituted with functional groups, to give a plurality of structurally distinct olefinic products. The methodology of the invention is also useful in facilitating the stereoselective synthesis of 1,2-disubstituted olefins in the cis configuration.
-
A recyclable CO surrogate in regioselective alkoxycarbonylation of alkenes: indirect use of carbon dioxide作者:P. H. Gehrtz、V. Hirschbeck、I. FleischerDOI:10.1039/c5cc05012j日期:——
Herein, we report a Pd-catalysed alkoxycarbonylation of alkenes based on the use of a recyclable CO2reduction product, the crystalline and air-stable
N -formylsaccharin, as a CO surrogate.在这里,我们报告了一种基于可回收的CO2还原产物的Pd催化烯烃羧酯化反应,该产物是结晶的、耐空气的N-甲酰糖脲,作为CO的替代物。 -
Salt suitable for an acid generator and a chemically amplified resist composition containing the same申请人:Harada Yukako公开号:US20070078269A1公开(公告)日:2007-04-05The present invention provides a salt of the formula (I): wherein ring Y represents monocyclic or polycyclic hydrocarbon group having 3 to 30 carbon atoms, in which one —CH 2 — group is substituted with —COO— group, and at least one hydrogen atom in the monocyclic or polycyclic hydrocarbon group may optionally be substituted with alkyl group having 1 to 6 carbon atom, alkoxy group having 1 to 6 carbon atom, perfluoroalkyl group having 1 to 4 carbon atoms, hydroxyalkyl group having 1 to 6 carbon atoms, hydroxyl group or cyano group; Q 1 and Q 2 each independently represent fluorine atom or perfluoroalkyl group having 1 to 6 carbon atoms; A + represents organic counter ion; and n shows an integer of 0 to 12. The present invention also provides a chemically amplified resist composition comprising the salt of the formula (I).
-
[EN] LIGHT HARVESTING ARRAY<br/>[FR] RÉSEAU COLLECTEUR DE LUMIÈRE申请人:FLUROSOL IND PTY LTD公开号:WO2015024064A1公开(公告)日:2015-02-26The invention relates to a light harvesting array or dye comprising an acceptor linked to a donor, wherein at least one of the acceptor or the donor is an oligomeric unit comprising a first optionally substituted rylene linked via a linker group to a second optionally substituted rylene, the first optionally substituted rylene is linked to the acceptor or the donor and the second optionally substituted rylene is capable of energy transfer to at least one of the first optionally substituted rylene, the acceptor or the donor. The invention also relates to compounds which may be used as light harvesting arrays, methods for their manufacture, and devices and materials comprising the light harvesting array or dye, for example, chromophoric materials, light guides, photobioreactors, photoluminescent algae systems, photodetectors, photovoltaic devices and luminescent/fluorescent solar concentrators.
-
Photocatalytic intermolecular carboarylation of alkenes by selective C–O bond cleavage of diarylethers作者:Meishan Ji、Chenyang Chang、Xinxin Wu、Chen ZhuDOI:10.1039/d1cc04038c日期:——Disclosed herein is a novel radical-mediated intermolecular carboarylation of alkenes by cleaving inert C–O bonds. The strategically designed arylbenzothiazolylether diazonium salts are harnessed as dual-function reagents. A vast array of alkenes are proven to be suitable substrates. The benzothiazolyl moiety in the products serves as the formyl precursor, and the OH residue provides the cross-coupling
表征谱图
-
氢谱1HNMR
-
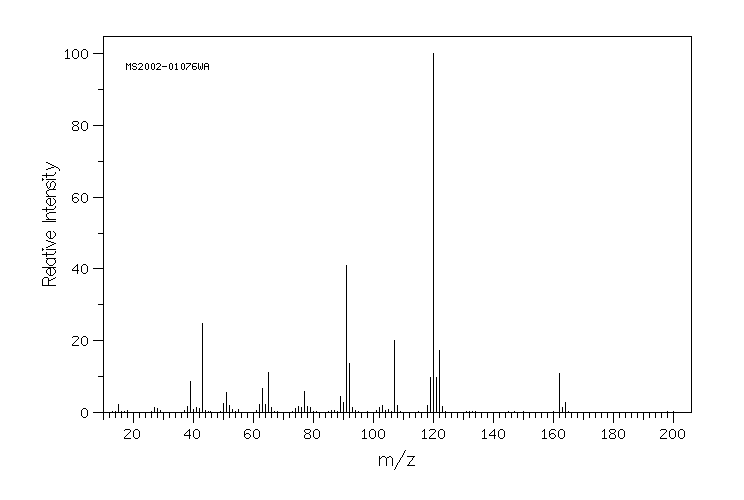
质谱MS
-
碳谱13CNMR
-
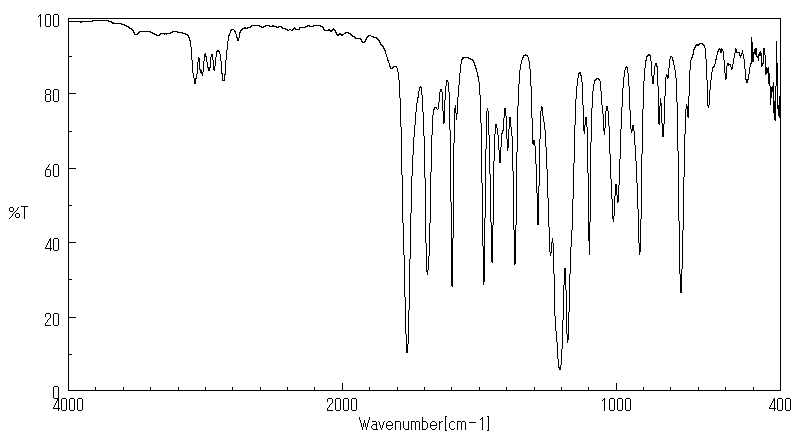
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰亚胺四聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺六聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺-酰胺-PEG8-四氟苯酚酯
马来酰亚胺-四聚乙二醇-五氟苯酯
马来酰亚胺-三聚乙二醇-五氟苯酚酯
靛酚乙酸酯
阿立哌唑标准品002
间硝基苯基戊酸酯
间氯苯乙酸乙酯
间乙酰苯甲酸
钾4-乙酰氧基苯磺酸酯
酚醛乙酸酯
邻苯二酚二乙酸酯
邻甲苯基环己甲酸酯
邻甲氧基苯乙酸酯
辛酸苯酯
辛酸对甲苯酚酯
辛酸五氯苯基酯
辛酸-(3-氯-苯基酯)
辛酰溴苯腈
苯酰胺,3,4-二(乙酰氧基)-N-[6-氨基-1,2,3,4-四氢-1-(4-甲氧苯基)-3-甲基-2,4-二羰基-5-嘧啶基]-
苯酚-乳酸
苯酚,4-异氰基-,乙酸酯(ester)
苯酚,4-[(四氢-2H-吡喃-2-基)氧代]-,乙酸酯
苯酚,3-(1,1-二甲基乙基)-,乙酸酯
苯酚,2-溴-3-(二溴甲基)-5-甲氧基-,乙酸酯
苯甲醇,4-(乙酰氧基)-3,5-二甲氧基-
苯甲酸,4-(乙酰氧基)-2-氟-
苯氧基氯乙酸苯酯
苯基金刚烷-1-羧酸酯
苯基氰基甲酸酯
苯基庚酸酯
苯基庚-6-炔酸酯
苯基己酸酯
苯基呋喃-2-羧酸酯
苯基吡啶-2-羧酸酯
苯基十一碳-10-烯酸酯
苯基乙醛酸酯
苯基乙酸酯-d5
苯基丙二酸单苯酯
苯基丙-2-炔酸酯
苯基丁-2,3-二烯酸酯
苯基4-乙基环己烷羧酸
苯基3-乙氧基-3-亚氨基丙酸盐
苯基2-(苯磺酰基)乙酸酯
苯基2-(4-甲氧基苯基)乙酸酯
苯基2-(2-甲氧基苯基)乙酸酯
苯基2-(2-甲基苯基)乙酸酯
苯基-乙酸-(2-甲酰基-苯基酯)
苯基-乙酸-(2-环己基-苯基酯)